CHEMISTRY
advertisement

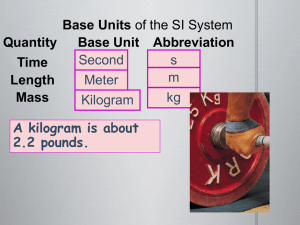
CHEMISTRY – Chapter 1 & 2 Matter, Measurements, and Calculations Chapter 1 – Section 1 Objectives: 1. Define chemistry 2. List examples of branches of chemistry 3. Compare and contrast basic research, applied research, and technological development What objects in this room are related to chemistry? Plastics Fabrics Clothes Cooking oil Motor oil Make-up Radio Batteries Computers Chemistry in our daily lives. Antibiotics Food Transportation Sports Farming Military Industry Chemistry Study of the composition and properties of matter and the changes that matter undergoes - What something is made of - What is the internal arrangement Chemical Any substance that has a definite composition 6 Main Branches of Chemistry 1. 2. 3. 4. 5. Organic – substances containing C Inorganic – substances other than organic Biochemistry – living things Physical chemistry – changes of matter Analytical chemistry – id components of materials 6. Theoretical chemistry – use math and computers to understand chemical behavior All branches involve some type of research. Basic research – to increase knowledge - how and why Applied research – to solve problems Technological development – production and use of products - lags behind discoveries - application of knowledge Review and Assignment 1. Define chemistry 2. List examples of branches of chemistry 3. Compare and contrast basic research, applied research, and technological development Assignment: WS 1-1 Quiz 1. Name two branches of chemistry. 2. List two ways that chemistry affects our daily lives. 3. Definition of chemistry. Chapter 1 - Matter Chapter 1 – Section 2 Objectives: 1. Distinguish between a mixture and a pure substance. 2. Define what matter is. Matter - anything that has mass and occupies space includes almost everything exceptions are light, heat, and sound properties are used to measure matter ex. mass Mass – measure of quantity of matter - not affected by temp, location, or any other factor Demo. Mass vs. matter What caused the change in mass? Is air matter? Matter (cont.) Classified into 2 groups: 1. pure substances 2. mixtures Pure substance – matter that has the same properties throughout ex. element or compound Pure Substances Element – substance that cannot be broken down by ordinary chemical change - only 1 type of atom - symbols abbreviated w/1 or 2 letters - can be an allotrope allotrope – one of a number of different molecular forms of an element in the same state Compound – substance made up of 2 or more elements chemically combined - can be broken down by chemical change - more than 1 type of atom Compounds 1. Elements that make up a compound are combined in definite proportion by mass ex. 100 g water has 11.2 g H and 88.8 g of O 2. Chemical and physical properties of compound differ from those of its parts ex. water is liquid, H and O are gases 3. Compounds can be formed from simpler substances by chem change and can be broken down into simpler substances example 100 of water has 11.2 g H and 88.8 g O How many g of H is in a 120g sample of water? 120 g water| 11.2 g H = 13.4 g H | 100 g water Mixtures - contain 2 or more substances that have - different properties - vary in composition and properties from sample to sample ex. rock, wood, salt water Not chemically combined - Can be separated by simple physical means - ie. filtration, evaporation, distillation Formation of Mixtures A mixture can be formed 3 ways: 1. Element mixed w/1 or more other elements ex. carbon w/sulfur 2. Compound mixed w/ 1 or more other compounds ex. salt w/sugar 3. 1 or more elements mixed w/1 or more compounds ex. sulfur w/sugar Characteristics of Mixtures - retain properties of each of its parts ex. iron and sulfur - iron remains magnetic - composition can vary widely - can be homogeneous or heterogeneous Types of mixtures Homogeneous – uniform composition throughout - called solutions ex. alloys, pop, air, coffee Heterogeneous – not uniform throughout ex. concrete, soil, dry soup, spaghetti and meat balls Matter Pure substance Element Compound Mixture Homogeneous Heterogeneous Review and Assignment 1. Distinguish between a mixture and a pure substance. 2. Define what matter is. Assignment: WS Chapter 1 – Section 2 Objectives: 1. Distinguish between the physical properties and chemical properties of matter. 2. Classify changes of matter as physical or chemical. 3. Explain the gas, liquid, and solid states in terms of particles. Properties of Matter - allow us to distinguish btwn substances - characteristics of a substance - what can be observed - way that a substance behaves ex. color, taste, odor, gas, liquid, solid Properties (cont.) - can be extensive or intensive Extensive – d/o amount of matter ex. volume, weight, mass, and E Intensive – does not d/o amount of matter ex. melting point, boiling point, density, and conductivity Demonstration Properties - water and glycerin How do they compare? - look, feel, weight, flow - water and salt water How do they compare? - conductivity Physical Properties Can be observed or measured w/out changing the substance Can describe the substance Odor, taste, hardness, density, melting point, and boiling point Metals – ductile (pulled into wire), malleable (hammered into sheets), luster (shine), good conductors Chemical Properties A transformation of a substance into a different one rusting, flammability, tarnishing, new substance formed Physical Change No new substance is formed CHANGE IN PHASE, pounding, grinding, cutting Changes of phase When a substance changes phase there is no change in composition Physically different, chemically the same Solid, liquid, or gas are the three states of matter States of Matter Solid – definite volume and shape Particles are in fixed positions Held w/strong attractive forces Liquid – definite volume and no definite shape Takes shape of container Particles can move past each other States of Matter (cont.) Gas – neither definite volume nor definite shape Particles move easily and are very far apart Plasma – high temperature state in which atoms lose their electrons Chemical Change One or more substance is changed to something new Rusting, burning, gas formed, digestion, heat or light added, explosion, color change, odor change, water formed Review and Assignment 1. Distinguish between the physical properties and chemical properties of matter. 2. Classify changes of matter as physical or chemical. 3. Explain the gas, liquid, and solid states in terms of particles. Assignment: p. 18 and WS CHEMISTRY – Chapter 1 – Section 3 Objectives: 1. Perform density calculations. 2. Describe conservation of mass. Properties of Matter - E is always involved in both physical and chemical changes - Physical are not at noticable - Chemical are more noticable - Heat and light are given off Density is a physical property is always the same for a solid substance in gases and some liquids a change in temperature will change the density increase in temperature will decrease density D = m/V Density problem Use the 5 steps in problem solving to solve the following problem. Lead has a mass of 22.7 g and its volume is 2.00 cm3. What is its density? m = 22.7 g V = 2.00 cm3 D = m/V = 22.7 g/2.00 cm3 = 11.4 g/ cm3 Examples Conservation of Mass In reactions matter cannot be created or destroyed by a chemical change - mass stays the same, it may just change form Density Lab Results Group 1 – Group 2 – Group 3 – Group 4 – Group 5 - Review and Assignment 1. Perform density calculations. 2. Describe conservation of mass. Assignment: WS and Density lab Chapter 2 - Sec.1 Objectives: 1. Describe the purpose of the scientific method. 2. Distinguish between qualitative and quantitative observations. 3. Describe the steps to making a graph. 4. Distinguish between inversely and directly proportional relationships. Scientific Method - a logical approach to solving problems 1. Make observations - observe your surroundings 2. State the problem - stated as a question 3. Collect data 4. Form hypothesis - testable statement 5. Test hypothesis 6. Conclusion 7. Modify hypothesis and retest Observing Involves making measurements and collecting data Data can be qualitative or quantitative Qualitative – non-numerical information - descriptive (the sky is blue) Quantitative – numerical information - the mass is 25.7 grams Conclusion Can be explained by using models Model – explanation of how phenomena occur or how things are related - visual - verbal - mathmatical Theory - models may become part a theory Theory – broad generalization that explains facts or phenomena - must be able to predict results ex. kinetic-molecular theory collision theory Controlled Experiments Use manipulated variable (independent) Use responding variable (dependent) One variable manipulated at a time Measurements are called data Making a Graph Shows results of an experiment in a meaningful pattern Dependent variable is on the vertical axis 1. Always include a title 2. Determine variables 3. Set up scale 4. Plot points 5. Draw best-fit line Oxygen obtained from electrolysis of water 3 10 18 23 28 oxygen (g) Oxygen Water 2.7 8.9 16 20.4 24.9 Electrolysis of water 25 20 15 10 5 0 28 23 18 10 3 0 5 10 15 water (g) 20 25 Relationships in graphs Directly proportional – if dividing one by the other gives you a constant value If one increases so does the other If started at point (0,0) Inversely proportional – if their product is constant If one increases the other decreases Produce a curve Review and Assignment 1. Describe the purpose of the scientific method. 2. Distinguish between qualitative and quantitative observations. 3. Describe the steps to making a graph. 4. Distinguish between inversely and directly proportional relationships. Assignment: graphing WS Quiz 1. List three steps of the scientific method. 2. List two steps in making a graph. Chapter 2 Sec.2 Objectives: 1. Distinguish between a quantity, a unit, and a measurement standard. 2. Name SI units for length, mass, time, volume, and density. 3. Distinguish between mass and weight. Measurements Basic part of science Make observations more meaningful Needs to be more than just a number or quantity Need a common system of units For consistency Measure your desk w/anything you have available SI System - The International System of Units Used in all science A standard Based on 10 - Makes it easier to convert from one unit to another SI System (continued) - 7 base units 1. Length – meter (m) 2. Mass – kilogram (kg) 3. Time – second (s) 4. Amount – mole (mol) 5. Temperature – Kelvin (K) 6. Electric Current – ampere (amp) 7. Luminous intensity – candela (cd) Weight vs. mass Mass – quantity of matter - how much space it takes up - measured w/a balance - unit kg Weight – F gravity pulls on matter with - measured w/spring scale - unit Newton On the moon will our weight or mass stay the same? SI Prefixes You must know these. Kilo- 1000 Deca – 10 Base unit (m, s, L) Centi – 1/100 or 0.01 Milli – 1/1000 0r 0.001 Derived Units - combination of base units Examples - Area = m2 - Volume = m3 - Density = kg/m3 - Newton = m٠kg/s2 Derived Units (cont.) Area – determined by multiplying 2 lengths Volume – determined by multiplying 3 lengths for a solid - for liquids unit is cm3 or mL ** 1 mL = 1 cm3 Review and Assignment 1. Distinguish between a quantity, a unit, and a measurement standard. 2. Name SI units for length, mass, time, volume, and density. 3. Distinguish between mass and weight. Assignment: WS 2-2 and p. 42 ~1-3 Quiz 1. 2. 3. 4. 5. What is the base SI unit for mass? Kilo = ______ Centi = _____ What is a derived unit? 1 cm3 = _____ mL Chapter 2 - Sec.3 Objectives: 1. Distinguish between accuracy and precision. 2. Determine the number of significant figures in measurements. 3. Perform mathematical operations involving significant figures. Accuracy and Precision Accuracy – closeness of a measurement to correct value Precision – closeness of a set of measurements to each other Consistency Do not have to be correct d/o measuring instrument Bullseyes Significant Figures - digits in a measurement that are know with certainty and one digit that is estimated - CALCULATORS DO NOT KEEP TRACK OF SIGNIFICANT FIGURES Significant Figure Rules 1. Digits other than zero are ALWAYS significant 2. 3. 4. 5. ex. 61.4 3 sig. fig. All zeros at the end of a number and to the right of the decimal with a # preceding the decimal are ALWAYS sig ex. 4.7200 km 5 sig. fig. Zeros used only for spacing are NOT significant ex. 7000 1 sig. fig. 20 1 sig. fig. 100.0 4 sig. fig. Zeros between sig. fig are significant Zeros in front of a non-zero are NOT sig. - don’t count until you get to 1st non-zero from lf to rt 0.004 1 sig. fig. 0.0009 1 sig. fig. Significant Figures 1,000 = _____ sig figs 100.0 = _____ sig figs 0.00012340 = _____ sig fig 10.0340 = _____ sig fig Calculating w/Significant Figures Addition and Subtraction - use same # of decimal places as the measurement w/the least decimal places ex. 2.098 3 DECIMAL places +6.2 1 DECIMAL place 8.298 round to 1 Decimal 8.3 is the final answer Adding and Subtracting 10.0 + 123 = _____ 23.456 – 23.0 = _____ 100.12 + 56.45 = _____ 1,000 + 12.234 = _____ Calculating w/sig. figs (cont.) Multiplication and Division - use same # sig. fig. as the measurement w/the least sig. fig. ex. 2.38 3 sig. fig x 9.0 2 sig. fig 21.42 round to 2 sig. Fig 21 is the final answer Multiplying and Dividing 100.0 x 10 = _____ 34.56 x 23.45 = _____ 12.045 x 34.008 = _____ 50.04 x 23 = _____ Review and Assignment 1. Distinguish between accuracy and precision. 2. Determine the number of significant figures in measurements. 3. Perform mathematical operations involving significant figures. Assignment: WS 2-6 and sig fig WS Quiz How many significant figures are in the following numbers? 1. 8,000 _____ 2. 100.01 _____ 3. 0.00056_____ 4. 4500.10 _____ 5. What is precision? Chapter 2 - Sec.3 Day 2 Objectives: 1. Perform mathematical operations involving percent error. Percent Error Observed value – based on lab measurements True value – based on generally accepted references Error exists in any measurement d/o measurer, instrument, conditions Percent Error % error = true value – obs. value x 100 true value Example atomic mass of Al = 28.9 g measured mass = 27.0 g What is the % error? 28.9 g – 27.0 g x 100 = 7.00 % 28.9 g Review and Assignment 1. Perform mathematical operations involving percent error. Assignment: WS 2-5 and % error WS Quiz How many significant figures are in the following numbers? 1. 8,104 _____ 2. 100.01 _____ 3. What does % error tell us? 4. What is accuracy? 5. What is precision? CHEMISTRY – Chapter 2 Sec.3 Day 3 Objectives: 1. Use dimensional analysis to convert measurements. 2. Convert measurements into scientific notation. 3. Perform mathematical operations using exponents. Problem Solving Rules Write down what is known. - mass = 346 g volume = 34.6 cm3 2. Write down unknown. - density = ? 3. Write the equation to use. D = m/V 4. Fill in knowns. D = 346 g/34.6 cm3 5. Solve for unknown and label. D = 200 g/cm3 6. Check your work. Dimensional Analysis - use with conversion factors to change from one unit to another Steps: convert 2550 m to km 1. Determine conversion factor - 1000 m to 1 km 2. Set up T-bars 3. Write given # in first box Dimensional Analysis (cont.) 4. Write conversion factor in 2nd box - unit on bottom matches unit of given # 5. Matching labels cancel - if 1 from conversion factor is on top divide - if 1 from conversion factor is on bottom multiple Scientific Notation - Used to represent very large or very small numbers - There are two parts - Basic form is M x 10n - M is a number - n is a number representing how many places to move the decimal Scientific Notation (cont.) If n is negative, your number is a decimal If n is positive, your number is a large number Examples: 60,000,000 = 6 x 107 0.000005 = 5 x 10-6 125,000 = 1.25 x 105 Scientific Notation (cont.) Write the following in scientific notation. 1,000,000,000 23,456 0.0005678 0.034 14,239.1 Scientific Notation (cont.) Write the following in long hand. 1. 1 x 10-9 2. 3.5 x 105 3. 7.123 x 10-3 4. 5 x 102 5. 4.56 x 10-2 Multiplication w/exponents Step 1 Multiply coefficients Step 2 Add exponents ex. (2 x 102) (2.5 x 105) = 5 x 107 Division w/exponents Step 1 Divide coefficients Step 2 Subtract exponents ex. (5 x 10-2) (1.0 x 107) = 5 x 10-9 Addition & Subtraction w/exponents All numbers must be written in the same power of 10 ex. 5.8 x 103 + 2.16 x 104 - change to 0.58 x 104 + 2.16 x 104 = 2.74 x 104 Scientific Notation & sig figs All numbers in front of the x 10 are significant ex. 2.00 x 102 = 3 sig fig 2 x 102 = 1 sig fig Scientific Notation & calculators 5.44 x 107/8.1 x 104 5.44 (EE or exp) 7 / 8.1 (EE or exp) 4 = 6.7 x 102 Review and Assignment 1. Use dimensional analysis to convert measurements. 2. Convert measurements into scientific notation. 3. Perform mathematical operations using exponents. Assignment: p. 57 ~ 1-7 and WS