Ch.18: Ethers and Epoxides; Thiols and Sulfides
Dr. Sivappa Rasapalli
Chemistry and Biochemistry
University of Massachusetts Dartmouth
Coverage and Objectives
Coverage:
• The nomenclature of alcohols
• Synthesis and Nucleophilic substitution reactions of ethers
• Nucleophilic Opening reactions of epoxides
• Chemistry of sulfur compounds
Learning objectives:
• Provide both IUPAC and common names for ethers, sulfides.
• Recognize the physical properties of ethers, epoxides and sulfides
• Know the synthesis and chemistry of the functional groups
• Write the reaction and electron-pushing (arrow-pushing)
mechanism for the synthesis and reactions of the functional groups
• Write the acid-catalyzed ring-opening of epoxides, and explain the
observed stereochemistry of the products.
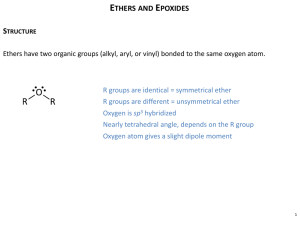
18.1 Names and Properties of Ethers
Nomenclature of Ethers, Epoxides, and
Sulfides
Ethers, Epoxides, Thiols and sulfides
•Ethers (ROR) can be regarded as derivatives of
alcohols (ROH).
•Sulfides (RSR) can be regarded as derivatives of
Thols (RSH).
Nomenclature: Functional Class
• simple ethers are named: “alkyl alkyl ether”
• name the groups attached to oxygen in alphabetical order as
separate words; "ether" is
last word;
• “dialkyl ether” if symmetric
Nomenclature: Substituent
• name as alkoxy derivatives of alkanes
CH3OCH2 CH3
methoxyethane
CH3CH2OCH2CH2CH2Cl
1-chloro-3-ethoxypropane
CH3CH2OCH2 CH3
ethoxyethane
Nomenclature: Functional Class
• analogous to ethers, but replace “ether” as last
word in the name by “sulfide.”
CH3SCH2 CH3
ethyl methyl sulfide
CH3CH2SCH2 CH3
diethyl sulfide
SCH3
cyclopentyl methyl sulfide
Nomenclature: Substituent
• name as alkylthio derivatives of alkanes
CH3SCH2 CH3
methylthioethane
SCH3
(methylthio)cyclopentane
CH3CH2SCH2 CH3
ethylthioethane
Names of Cyclic Ethers
O
Oxirane
(Ethylene oxide)
O
Oxetane
O
Oxolane
(tetrahydrofuran)
O
O
Oxane
(tetrahydropyran)
O
1,4-Dioxane
Names of Cyclic Sulfides
S
S
Thiirane
Thietane
S
Thiane
S
Thiolane
Examples of Nomenclature
Br
O
OCH3
but yl ethyl et her (common)
or
1-ethoxybut ane (IUP AC)trans 1-bromo-2-methoxycyclopent ane
O
CH3CH2O
OH
CH3CH2O
ethyl (Z) 1-propenyl ether (common)
or
(Z) 1-et hoxy-1-propene (IUP AC)4,4-diethoxy-2-cyclohexenol
O
OCH3
O
cis 3-met hoxycyclopentene oxide
(E) 2-met hyl-3-hexene oxide
trans 2-et hyl-3-isopropyloxirane
(E) 2-met hyl-3,4-epoxyhexane cis 3-met hoxy-1,2-epoxycyclopent ane
Periplenone B
Epothilone B
female cockroach sex pheromone ant icancer drug from soil bacteria
O
O
O
S
O
H
OH
N
O
O
OH
O
Structure and Bonding
in
Ethers and Epoxides
Bond angles at oxygen are sensitive
to steric effects
O
O
H
H
105°
H
CH3
108.5°
O
O
CH3
CH3
112°
(CH3)3C
C(CH3)3
132°
An oxygen atom affects geometry in
much the
same way as a CH2 group
most stable conformation of diethyl ether
resembles pentane
An oxygen atom affects geometry in much the
same way as a CH2 group
most stable conformation of tetrahydropyran
resembles cyclohexane
Physical Properties of Ethers
• Boiling points (and melting points) of ethers are lower than
corresponding alcohol
– E.g. CH3CH2OH (bp 78°C) vs. CH3-O-CH3 (bp -25°C)
• Why? No Hydrogen-bonding for ethers
Ethers resemble alkanes more than alcohols
with respect to boiling point
boiling point
36°C
35°C
O
OH
117°C
Intermolecular hydrogen
bonding possible in
alcohols; not possible
in alkanes or ethers.
Ethers resemble alcohols more than alkanes
with respect to solubility in water
solubility in water (g/100 mL)
very small
O
7.5
Hydrogen bonding to
water possible for ethers
and alcohols; not
possible for alkanes.
9
OH
• Solubility of acyclic ethers in water is less than that of
corresponding alcohols of equal MW.
– Ethers can accept H-bonds, but not donate them
Physical Properties-Uses
Ethers are less dense than water and form the top layer when mixed
with water.
• Note: Diethyl ether (“ether”) is a good general purpose solvent for
extracting non-polar and polar organic compounds from H2O.
• Its low boiling pt (35 oC) is ideal for recovering organic solute by
evaporation of ether.
Solubility of cyclic ethers in water is greater than that of acyclic ethers
of equal MW.
• Compact shape more easily accommodated by H-bonding network of
water ; Ex: Tetrahydrofuran 1,4-Dioxane completely miscible with water;
important cosolvents
Solvation
Crown Ethers
• structure
cyclic polyethers derived from repeating —OCH2CH2— units
• properties
form stable complexes with metal ions
• applications
synthetic reactions involving anions
O
O
O
O
O
O
18-Crown-6
18-Crown-6
O
O
O
K+
O
O
O
forms stable Lewis acid/Lewis base complex with K+
Ion-Complexing and Solubility
+ F–
O
O
O
O
O
O
K+
O
O
O
benzene
O
O
O
18-crown-6 complex of K+ dissolves in benzene
F– carried into benzene to preserve electroneutrality
Application to organic synthesis
Complexation of K+ by 18-crown-6 "solubilizes"
potassium salts in benzene
Anion of salt is in a relatively unsolvated state in
benzene (sometimes referred to as a "naked anion")
Unsolvated anion is very reactive
Only catalytic quantities of 18-crown-6 are needed
KF
CH3(CH2)6CH2Br
18-crown-6
benzene
CH3(CH2)6CH2F
(92%)
18. 2 Synthesis of Ethers
Acid–catalyzed dehydration of Alcohols
Diethyl ether prepared industrially by sulfuric acid–
catalyzed dehydration of ethanol – also with other
primary alcohols
The Williamson Ether Synthesis
Reaction of metal alkoxides and primary alkyl halides
and tosylates
Best method for the preparation of ethers
Alkoxides prepared by reaction of an alcohol with a
strong base such as sodium hydride, NaH
Example
ONa
O
I
NaI
RO-, an alkoxide ion, is both a strong nucleophile (unless bulky and hindered)
and a strong base. Both SN2 (desired) and E2 (undesired side product) can
occur.
• Choose nucleophile and electrophile carefully. Maximize SN2 and
minimize E2 reaction by choosing the R’X to have least substituted carbon
undergoing substitution (electrophile). Methyl best, then primary, secondary
marginal, tertiary never (get E2 instead).
• Stereochemistry: the reacting carbon in R’, the electrophile which
undergoes substitution, experiences inversion. The alkoxide undergoes no
change of configuration.
Williamson’s Ether Synthesis has Limitations
1. Alkyl halide must be primary (RCH2X)
2. Alkoxides need be derived from primary, secondary or tertiary alcohols
O Na
OH
Secondary
Alkoxide
SN2
This reaction worlks particularly
well with benzyl and allyl
halides, which are excellent
alkylating agents
Br
O
Primary
Alkyl halide
Origin of Reactants
OH HCl
Cl
O
OH
O Na
Na
What happens if the alkyl halide is not primary?
Br
O Na
H3C
CH3
SN2 is sterically
disfavored and E2
predominates
O
H
OH
H3C
H
Silver Oxide-Catalyzed Ether Formation
Reaction of alcohols with Ag2O directly with alkyl halide
forms ether in one step
Glucose reacts with excess iodomethane in the
presence of Ag2O to generate a pentaether in 85% yield
Addition of Alcohols to Alkenes
(CH3)2C=CH2 + CH3OH
H+
(CH3)3COCH3
tert-Butyl methyl ether
tert-Butyl methyl ether (MTBE) was produced on a scale exceeding 15 billion
pounds per year in the U.S. during the 1990s. It is an effective octane booster
in gasoline, but contaminates ground water if allowed to leak from storage
tanks. Further use of MTBE is unlikely.
Alkoxymercuration
Mechanism of Oxymercuration
Reactions of Ethers:
Summary of reactions of ethers
No reactions of ethers encountered to this point.
Ethers are relatively unreactive.
Their low level of reactivity is one reason why ethers
are often used as solvents in chemical reactions, and as
protecting groups for reactive –OH group.
Ethers oxidize in air to form explosive hydroperoxides
and peroxides.
Acid-Catalyzed Cleavage of Ethers
Ethers are generally unreactive
Strong acid will cleave an ether at elevated temperature
HI, HBr produce an alkyl halide from less hindered
component by SN2 (tertiary ethers undergo SN1)
Example
CH3CHCH2CH3 HBr
OCH3
heat
CH3CHCH2CH3
Br
(81%)
+ CH3Br
Mechanism
CH3CHCH2CH3
CH3CHCH2CH3
O ••
Br
CH3 ••
H
••
Br ••
HBr
••
CH3CHCH2CH3
CH3CHCH2CH3
•• –
•• Br ••
••
O
CH3 ••
+
H
•• O
••
••
•• Br
••
H
CH3
Cleavage of Cyclic Ethers
O
HI
150°C
ICH2CH2CH2CH2I
(65%)
Mechanism
••
ICH2CH2CH2CH2I
O
••
HI
•• –
•• I •
•
••
HI
••
O+
H
••
•• I
••
••
•• O
H
Claisen Rearrangement
• Specific to allyl aryl ethers, ArOCH2CH=CH2
• Heating to 200–250°C leads to an o-allylphenol
• Result is alkylation of the phenol in an ortho position
Epoxides (Oxiranes)
• Three membered ring ether is called an oxirane (root “ir” from
“tri” for 3-membered; prefix “ox” for oxygen; “ane” for
saturated)
• Also called epoxides
• Ethylene oxide (oxirane; 1,2-epoxyethane) is industrially
important as an intermediate
• Prepared by reaction of ethylene with oxygen at 300 °C and
silver oxide catalyst
Epoxides are Extremely Reactive
Preparation of Epoxides Using a Peroxyacid
• Treat an alkene with a peroxyacid
O
O
RCOOH
H
O
H
in CH2Cl2
R
C
O
O
Cl
H
O
CO3H
MCPBA
meta chloroperoxybenzoic acid
+ RCOH
Epoxide Ring Opening reactions:
1. Epoxide Ring Opening in Acid
In acid: protonate the oxygen, establishing the very good leaving
group. More substituted carbon (more positive charge although more
sterically hindered) is attacked by a weak nucleophile.
Very similar to opening
of cyclic bromonium ion.
Review that subject.
Due to
resonance,
some positive
charge is
located on
this carbon.
Inversion
occurs at this
carbon. Do you
see it?
Classify the
carbons. S
becomes R.
2. Epoxide Ring Opening in Base
In base: no protonation to produce good leaving group, no resonance but
the ring can open due to the strain if attacked by good nucleophile. Now
less sterically hindered carbon is attacked.
A wide variety of synthetic uses can be
made of these reactions as shown in the
following slides
Base-Catalyzed Epoxide Opening
• Strain of the three-membered ring is relieved on ring-opening
• Hydroxide cleaves epoxides at elevated temperatures to give
trans 1,2-diols
In base: no protonation to
OH
O
NaOCH3 in CH3OH
OCH3
produce good leaving group, no
resonance but the ring can open
H
OCH3
due to the strain if attacked by
O Na
OCH3
regenerates base catalyst
good nucleophile. Now less
sterically hindered carbon is
attacked.
18.6 Reactions of Epoxides: Ring-Opening
• Water adds to epoxides with dilute acid at room temperature
• Product is a 1,2-diol (on adjacent C’s: vicinal)
• Mechanism: acid protonates oxygen and water adds to
opposite side (trans addition)
Halohydrins from Epoxides
• Anhydrous HF, HBr, HCl, or HI combines with an epoxide
• Gives trans product
Epoxides from Halohydrins
• Addition of HO-X to an alkene gives a halohydrin
• Treatment of a halohydrin with base gives an epoxide
• Intramolecular Williamson ether synthesis
O
OH
Br2
+ HBr
Br
Addition of Grignards to Ethylene Oxide
• Adds –CH2CH2OH to the Grignard reagent’s hydrocarbon
chain
• Acyclic and other larger ring ethers do not react
O
RCO3H
CH3MgBr
OH
MgBrO
CH3
+ enant .
H 3O
+
CH3
Different isomers
Variety of products can be obtained by
varying the nucleophiles
H2O/ NaOH
Do not memorize
this chart. But be
sure you can figure
it out from the
general reaction:
attack of
nucleophile in
basic media on
less hindered
carbon
1.
2.
OH
LiAlH4
H2O
An Example of Synthetic Planning
Reactions of a nucleophile (basic) with an epoxide/oxirane ring
reliably follow a useful pattern.
The epoxide
ring has to
have
been
located here
The pattern to
be recognized in
the product is
–C(-OH) – C-Nu
This
bond
was created
by
the
nucleophile
Synthetic Applications
nucleophile
Realize that the H2NCH2was derived from
nucleophile: CN
N used as
nucleophile
twice.
Formation of ether from
alcohols.
Epichlorohyrin and Synthetic Planning, same as
before but now use two nucleophiles
Observe the pattern in the product
Nu - C – C(OH) – C - Nu. When you observe
this pattern it suggests the use of epichlorohydrin.
Both of these bonds will
be formed by the
incoming nucleophiles.
Why does Acid Catalyzed Opening Give
Inversion?
O
CH3CH2
CH3
NaOH, H2O
(S)
+
H 3O
HO
CH3CH2
CH3
CH2OH
(S)
HO
CH3
CH3CH2
CH2OH
(R)
Propose a Mechanism
Br
O
1) NaOCH3
2) heat
CH3OCH2
O
+ NaBr
2 Successive SN2 Reactions
OCH3
Br
1) NaOCH3
2) heat
O
Br
CH3O
O
CH3OCH2
O
+ NaBr
Provide a Mechanism
OH
+
H , H2O
O
OH
• Sulfur-containing amino acids
• • Methionine and Cysteine
• Important in protein secondary and tertiary structure
H
N
O
S
N
O
penicillin
H
N
H
S
MeO
O O
S
O
N
N
H2N
CO2H
N
H
OMe
omeprazole
dapsone
SO3 Na
Physical Properties
• Thiols and sulfides are more volatile (lower bp) than
corresponding alcohols or ethers.
Stench! Skunky!
• Hydrogen bonding less important for thiols compared to
alcohols
• Dipoles are less pronounced
Thiols are more acidic than alcohols (pKa)
– Size mismatch between small hard proton and large,
polarizable sulfur atom favours formation of thiolate anion.
Thiols: Formation and Reaction
• From alkyl halides by displacement with a sulfur nucleophile
such as –SH
– The alkylthiol product can undergo further reaction with
the alkyl halide to give a symmetrical sulfide, giving a
poorer yield of the thiol
Using Thiourea to Form Alkylthiols
• Thiols can undergo further reaction with the alkyl halide to
give dialkyl sulfides
• For a pure alkylthiol use thiourea (NH2(C=S)NH2) as the
nucleophile
• This gives an intermediate alkylisothiourea salt, which is
hydrolyzed cleanly to the alkyl thiourea
Preparation of Thiols
Use an alkyl halide and H2S or AcSH
H2S, KOH, EtOH
Br
N
Ph
SH
N
i, (COCl)2, DMF, CH2Cl2, r.t.
OH
ii, KSAc, DMF, r.t.
iii, KOH, EtOH, r.t. then HCl pH 5
Ph
SH
J. Am. Chem. Soc., 2005, 127, 15668
OH
OH
NH2
Br
SH
i, NaNO2, HCl
ii, KS
OEt , H2O
Br
S
iii, LiAlH4
J. Org. Chem., 1990, 55, 2736
For palladium coupling of AcS– and ArX, see Tetrahedron Lett., 2007, 48, 3033
Oxidation of Thiols to Disulfides
• Reaction of an alkyl thiol (RSH) with bromine or iodine gives
a disulfide (RSSR)
• The thiol is oxidized in the process and the halogen is
reduced
Sulfides
• Thiolates (RS) are formed by the reaction of a thiol with a
base
• Thiolates react with primary or secondary alkyl halide to give
sulfides (RSR’)
• Thiolates are excellent nucleophiles and react with many
electrophiles
Sulfides as Nucleophiles
• Sulfur compounds are more nucleophilic than their oxygencompound analogs
– 3p electrons valence electrons (on S) are less tightly held
than 2p electrons (on O)
• Sulfides react with primary alkyl halides (SN2) to give
trialkylsulfonium salts (R3S+)
Sulfides as Nucleophiles
Oxidation of Sulfides
• Sulfides are easily oxidized with H2O2 to the sulfoxide
(R2SO)
• Oxidation of a sulfoxide with a peroxyacid yields a sulfone
(R2SO2)
• Dimethyl sulfoxide (DMSO) is often used as a polar aprotic
solvent
Oxidation of Sulfides
Reaction of Thiols
Good nucleophiles
BuBr, Cs2CO3
SH
S
DMF, Bu4NI, r.t.
93%
HO
PhCH2Cl, Cs2CO3
SH
HO
DMF, Bu4NI, r.t.
S
Ph
73%
MeO
BrCH2CO2tBu, Cs2CO3
SH
DMF, Bu4NI, r.t.
MeO
S
CO2tBu
97%
Tetrahedron Lett., 2005, 46, 8931
Suitable for alkyl-alkyl and aryl-alkyl thioethers
Successful for secondary alkyl iodides (iPrI) but not tertiary halides
No racemization using L-cysteine methyl ester
18.9 Spectroscopy of Ethers
• Infrared: C–O single-bond stretching 1050 to 1150 cm1
overlaps many other absorptions.
• Proton NMR: H on a C next to ether O is shifted downfield to
3.4 to 4.5
– The 1H NMR spectrum of dipropyl ether shows this signal
at 3.4
– In epoxides, these H’s absorb at 2.5 to 3.5 d in their 1H
NMR spectra
• Carbon NMR: C’s in ethers exhibit a downfield shift to 50 to
80