WP4 - Hughes
advertisement

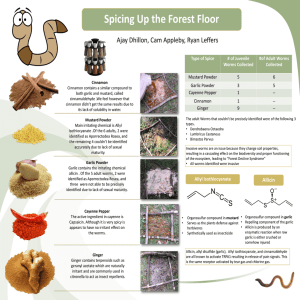
The Biosynthesis of Alliin Jill Maria Hughes with Hamish Collin, Rick Cosstick, Meriel Jones, Brian Tomsett and Angela Tregova EU Project No.QLK1-CT-1999-00498 Biochemistry of Garlic at Liverpool How is Alliin synthesised Where is it synthesised? When is it synthesised? Biosynthetic Pathway SO42serine SO32- Allyl group (unknown sources) SO22valine & methacrylate S-allylglutathione cysteine glutathione (γ-glu-cys-gly) S-(2-carboxypropyl)-glutathione gly S-allyl-γ-glu-cys S-methylglutathione gly S-2-CP-γ-glu-cys gly S-methyl-γ-glu-cys HCOOH glu transpeptidase S-allylcysteine S-allylcysteine oxidase oxidase alliin S-allyl-cysteine sulphoxide (alliin) S-trans-1-propenyl-γ-glu-cys glu transpeptidase glu transpeptidase S-methylcysteine S-trans-1-propenylcysteine oxidase S-trans-1-propenylcysteine sulphoxide (isoalliin) oxidase methiin The Serine and Glutathione Pathways Cysteine Unknown Allyl source Serine Glutathione Allyl Glutathione Serine pathway Gamma-Glutamyl Allyl Cysteine Allyl Cysteine Alliin Glutathione pathway Our Approach this year Precursor feeding. Modify method from isocratic to gradient. Use HPLC to identify intermediates. Allyl Cysteine Synthase. Identification of a specific garlic enzyme that can synthesise Allyl Cysteine. HPLC Method Development To aid identification of cysteine conjugates, our simple isocratic HPLC Method has been adapted: •Heptane sulphonic acid method was tried but was not suitable for indentification of compounds by Mass Spectroscopy •We developed a simple HCl:Acetonitrile gradient which allows simultaneous identification of cysteine sulphoxides and gammaglutamyl peptides. •This method is now in routine use in our laboratory. HPLC Method Development Alliin Gamma-glutamylallylcysteine Isoalliin Typical HPLC trace of Garlic clove gradient HPLC Method Development Standards Peak Identification: •Compare unknown peak retention times with standards •Mass spectroscopyidentify from ‘general profile’ and mass of molecular ion Methyl CSO Ethyl CSO Methyl cysteine Alliin Propenyl CSO Propyl CSO Glutathione Gamma glutamyl cysteine Methionine Ethyl cysteine Methyl glutathione 3-CPC 2-CPC Allyl cysteine Butyl CSO 1,2 Dicarboxy glutathione Propenyl cysteine propyl cysteine Gamma glutamyl allyl cysteine Butyl cysteine Propyl glutathione Rt 0.9ml/min 4.25 3.76 4.25 4.49 5.24 5.76 6.01 6.78 6.74 7.04 10.11 10.56 11.74 11.69 12.06 12.53 14.66 15.44 20.19 21.86 20.8 Synergi RPMax 100%0.03MHCl@5min 50%Acetonitrile@25min HPLC Method Development Alliin Gamma-glutamylallylcysteine Gamma-glutamylisoallylcysteine Isoalliin Typical HPLC trace of Garlic clove gradient Precursor Feeding Experiments Using single clone garlic callus to reduce variation In Allium callus tissue the general reaction seems to apply: Alk(en)yl thiol Alk(en)yl cysteine Alk(en)yl CSO Garlic GAt2b Alliin Propiin Allyl cysteine Propyl cysteine GPt2a 41 12 81 5 GAt6b Alliin Propiin Allyl cysteine Propyl cysteine GPt6c 0 0 36 0 GAt13a Alliin Propiin Allyl cysteine Propyl cysteine 42 135 0 85 GAC6a 56 239 34 161 GPt13b 0 0 0 0 GAC2b GPC2c Av GBl2 509 6 14 14 26 3 198 0 0 12 138 0 23 203 0 60 22 14 348 8 GPC6a 24 61 27 248 Av GBl6 20 4 0 6 GAC13b GPC13b Av GBl13 53 30 14 6 167 12 302 0 0 0 254 11 Onion Alliin Propiin Allyl cysteine Propyl cysteine OnAt2c OnPt2a OnAC2a OnPC2b Av OnBl2 146 12 172 80 77 9 31 27 30 10 196 0 446 0 0 23 80 25 170 14 Alliin Propiin Allyl cysteine Propyl cysteine OnAt6c OnPt6a OnAC6a OnPC6b Av OnBl6 131 74 586 109 88 24 103 9 325 13 286 65 716 0 0 23 163 0 278 30 Alliin Propiin Allyl cysteine Propyl cysteine OnAt13c OnPt13b OnAC13b OnPC13b Av OnBl13 34 12 348 57 53 12 106 11 171 16 153 0 349 0 0 9 21 8 188 15 Precursor Feeding ExperimentsBoth Garlic and Onion callus can convert exogenously applied allyl thiol and propyl thiol to their respective cysteine conjugate and subsequently to their respective cysteine sulphoxide(CSO) Alliin 100 80 Allyl Cysteine Allyl thiol 60 40 Onion callus incubated with allyl thiol and propyl thiol for six days. 20 0 0.00 100 10.00 20.00 Propiin 80 30.00 Propyl cysteine 60 40.00 Propyl thiol 40 20 0 0.00 10.00 20.00 30.00 40.00 100 80 Control 60 40 20 0 0.00 10.00 20.00 30.00 40.00 Is there an Allyl Cysteine Synthase? Some purified plant cysteine synthase enzymes have shown the capability of producing cysteine conjugates in addition to their normal function Is there a specific cysteine synthase homologue in garlic that can conjugate allyl thiol to (O-acetyl)serine to give allyl cysteine and subsequently alliin? Cysteine synthase Sulphide + O-Acetyl Serine Cysteine Allyl thiol + O-Acetyl Serine Allyl Cysteine Allyl Cysteine synthase Alliin The Serine and Glutathione Pathways Cysteine Serine Serine pathway Unknown Allyl source Glutathione Allyl Glutathione Allyl Cysteine synthase Allyl Cysteine Alliin Gamma-Glutamyl Allyl Cysteine Glutathione pathway Protein purification: Ion Exchange chromatography Garlic leaves were fractionated with ammonium sulphate then separated by ion-exchange chromatography. Cysteine synthase activity. Q-Sepharose. 7.11.01 0.3 0.25 0.2 0.15 0.1 0.05 0 Fr 1 Fr 5 Fr 9 Fr 13 Fr 17 Fr 21 Fr 25 Fr 29 Fr 33 Only a few fractions show allyl cysteine synthase activity OD Many fractions show cysteine synthase activity cysteine synthase activity Fraction Protein purification: Hydrophobic Interaction Chromatography 0.6 0.5 0.4 0.3 0.2 cysteine 0.1 syntase activity Fraction 39 37 35 33 31 29 27 25 23 21 19 17 15 13 11 9 7 5 allyl cysteine synthase activity 3 0 1 Cysteine production was assayed colorimetrically and allyl cysteine by HPLC 0.7 OD550 Allyl cysteine synthase and cysteine synthase activity co-elute Phenyl sepharose fractionation Protein purification: How pure is the Fraction? SDS-PAGE shows a distinct band in the allyl cysteine synthase active fractions at approx. 34000 kdal. This M Wt. is consistent with plant cysteine synthase monomers found previously 34000 Fractions 26 27 28 29 30 What is the Enzyme? The selected 34000 band was digested with trypsin and the resultant peptides separated by preparative HPLC. Three selected peptides were sequenced:…….FLGVMPSHYSIE………. YLGADLALTDTN………… SANPGAHYATTGP…………. A simple BLAST search of these peptides in the protein database shows most similarity to a cysteine synthase from Oryza sativa (Rice) Biochemistry of Garlic at Liverpool Where now? This enzyme will be selected and overexpressed Enyme kinetics of the purified enzyme should help to clarify it’s role in vivo. The future? Use similar techniques to probe the glutathione pathway? The Serine and Glutathione Pathways Cysteine Serine Serine pathway Unknown Allyl source Glutathione Allyl Glutathione Allyl Cysteine synthase Allyl Cysteine Alliin Glutathione-STransferase Gamma- Glutamyl Allyl Cysteine Glutathione pathway The Biosynthesis of Alliin Jill Maria Hughes Acknowledgements: Mark Prescott Mass Spectroscopy Mark Wilkinson Protein purification facilities and Peptide Sequencing EU Project No.QLK1-CT-1999-00498