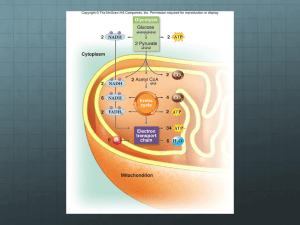
Cellular Respiration
advertisement

Making energy! ATP The point is to make ATP! Chemical energy First Law Of Thermodynamics (a) First law of thermodynamics: Energy can be transferred or transformed but Neither created nor destroyed. For example, the chemical (potential) energy in food will be converted to the kinetic energy of the cheetah’s movement in (b). Figure 8.3 Heat Second Law co2 + H2O (b) Second law of thermodynamics: Every energy transfer or transformation increases the disorder (entropy) of the universe. For example, disorder is added to the cheetah’s surroundings in the form of heat and the small molecules that are the by-products of metabolism. Figure 8.3 Free Energy Reactions in a Closed System: ∆G < 0 ∆G = 0 What would happen To a living System if it were closed? Body Cells: ∆G < 0 What do we Need to Stay alive? The energy needs of life Organisms are endergonic systems What do we need energy for? synthesis building biomolecules reproduction movement active transport temperature regulation Where do we get the energy from? Work of life is done by energy coupling use exergonic (catabolic) reactions to fuel endergonic (anabolic) reactions digestion + synthesis + + energy + energy ATP Adenosine TriPhosphate modified nucleotide nucleotide = adenine + ribose + Pi AMP AMP + Pi ADP ADP + Pi ATP adding phosphates is endergonic How efficient! Build once, use many ways high energy bonds How does ATP store energy? ADP AMP ATP I think he’s a bit unstable… don’t you? O– O– O – O– O– –O P –O O– P –O O––P OO P––O O– P O– O O O O O Each negative PO4 more difficult to add Instability of its P bonds makes ATP an excellent energy donor How does ATP transfer energy? ADP ATP O– O– O – –O P –O O– P –O O– P O– O O O O– –O P O – + O 7.3 energy ATP ADP releases energy ∆G = -7.3 kcal/mole Fuel other reactions Phosphorylation released Pi can transfer to other molecules destabilizing the other molecules enzyme that phosphorylates = “kinase” An example of Phosphorylation… Building polymers from monomers need to destabilize the monomers phosphorylate! H C OH + H C HO H C It’s never that OH simple! + ATP H C + P H C HO synthesis +4.2 kcal/mol “kinase” enzyme -7.3 kcal/mol -3.1 kcal/mol enzyme H H C C O H C H H C C OHHO + + H2O ADP P H H C C O + Pi Cells spend a lot of time making ATP! The point is to make ATP! What’s the point? How is ATP Made in a Cell? Substrate Level Phosphorylation Chemiosmosis Start with a mitochondrion or chloroplast Trap H+ in the intermembrane space Chemiosmosis Start with a mitochondrion or chloroplast Trap H+ in the intermembrane space How can this lead to ATP production? H+ ATP synthase Enzyme channel in H+ H+ H+ H+ H+ H+ H+ rotor mitochondrial membrane permeable to H+ H+ flow down concentration gradient rod flow like water over water wheel flowing H+ cause ADP + P change in shape of ATP synthase enzyme powers bonding of ATP Pi to ADP: ADP + Pi ATP But… How is the proton (H+) gradient formed? catalytic head H+ That’s the rest of my story! Any Questions? Cellular Respiration Harvesting Chemical Energy ATP What’s the point? The point is to make ATP! ATP Harvesting stored energy Energy is stored in organic molecules carbohydrates, fats, proteins Heterotrophs eat these organic molecules food Which releases more Energy, combustion Of glucose or Cellular respiration? respiration Harvesting stored energy glucose + oxygen energy + water + carbon dioxide C6H12O6 + 6O2 ATP + 6H2O + 6CO2 + heat COMBUSTION = making a lot of heat energy by burning fuels in one step fuel carbohydrates) RESPIRATION = making ATP (& some heat) by burning fuels in many small steps ATP enzymes O2 ATP O2 CO2 + H2O + ATP (+ heat) glucose CO2 + H2O + heat How do we harvest energy from fuels? Oxidation Reduction loses e- gains e- + oxidized reduced + + eoxidation e- – ereduction redox How do we move electrons in biology? Moving electrons in living systems electrons cannot move alone in cells electrons move as part of H atom e p move H = move electrons loses e- gains e- oxidized + + oxidation reduced + – H reduction H oxidation C6H12O6 + H e- 6O2 6CO2 + 6H2O + ATP reduction like $$ in the bank Moving electrons in respiration Electron carriers move mighty electrons by shuttling H atoms around NAD+ NADH (reduced) FAD+2 FADH2 (reduced) NAD+ nicotinamide Vitamin B3 niacin O– O – P –O O phosphates O– O – P –O O H reducing power! NADH O H H C NH2 N+ + adenine ribose sugar C NH2 reduction O– – – oxidation O P O O O– O – P –O O carries electrons as H O a reduced molecule N+ How efficient! Build once, use many ways Overview of cellular respiration 4 metabolic stages Anaerobic respiration 1. Glycolysis respiration without O2 in cytosol Aerobic respiration respiration using O2 in mitochondria 2. Pyruvate oxidation 3. Krebs cycle 4. Electron transport chain C6H12O6 + 6O2 ATP + 6H2O + 6CO2 (+ heat) Cellular Respiration Stage 1: Glycolysis Glycolysis Breaking down glucose “glyco – lysis” (splitting sugar) glucose pyruvate 2x 3C 6C In the cytosol? Why does that make evolutionary sense? but it’s inefficient generate only 2 ATP for every 1 glucose still is starting point for ALL cellular respiration occurs in cytosol That’s not enough ATP for me QuickTime™ and a decompressor are needed to see this picture. Evolutionary perspective Enzymes of glycolysis are “well-conserved” Prokaryotes first cells had no organelles Anaerobic atmosphere life on Earth first evolved without free oxygen (O2) in atmosphere Prokaryotes that evolved glycolysis are ancestors of all modern life ALL cells still utilize glycolysis You mean we’re related? Do I have to invite them over for the holidays? Overview glucose C-C-C-C-C-C 10 reactions enzyme 2 ATP enzyme 2 ADP fructose-1,6bP P-C-C-C-C-C-C-P enzyme enzyme enzyme What has more Free energy, G3P or pyruvate? DHAP P-C-C-C G3P C-C-C-P 2H 2Pi enzyme 2 NAD+ 2 enzyme 2Pi 4 ADP enzyme pyruvate C-C-C 4 ATP Glycolysis summary endergonic invest some ATP ENERGY INVESTMENT -2 ATP ENERGY PAYOFF G3P C-C-C-P 4 ATP exergonic harvest a little ATP & a little NADH like $$ in the bank NET YIELD net yield 2 ATP 2 NADH Substrate-level Phosphorylation In the last steps of glycolysis, where did the P come from to make ATP? 9 the sugar substrateH O(PEP) enolase OH2O 2 P is transferred from PEP to ADP kinase enzyme ADP ATP Phosphoenolpyruvate (PEP) ADP Phosphoenolpyruvate (PEP) 10 pyruvate kinase Pyruvate What sort of Enzyme does This? Pyruvate C CH2 O O OC ATP ATP ATP ADP C O C O CH3 P Energy accounting of glycolysis 2 ATP 2 ADP glucose pyruvate 2x 3C 6C 4 ADP 4 ATP 2 NAD+ 2 Net gain = 2 ATP + 2 NADH some energy investment (-2 ATP) small energy return (4 ATP + 2 NADH) 1 6C sugar 2 3C sugars The magic number is? Where is the Extra energy? Is that all there is? Not a lot of energy… for 1 billon years+ this is how life on Earth survived no O2 = slow growth, slow reproduction only harvest 3.5% of energy stored in glucose more carbons to strip off = more energy to harvest O2 O2 O2 O2 O2 glucose pyruvate 2x 3C 6C Hard way to make a living! But can’t stop there! G3P DHAP NAD+ raw materials products Pi + NADH NAD NADH Pi 6 1,3-BPG NAD+ Pi + NADH NAD 1,3-BPG NADH 7 ADP Glycolysis Pi ADP ATP ATP 3-Phosphoglycerate (3PG) 3-Phosphoglycerate (3PG) 2-Phosphoglycerate (2PG) 2-Phosphoglycerate (2PG) glucose + 2ADP + 2Pi + 2 NAD+ 2 pyruvate + 2ATP + 2NADH 8 Going to run out of NAD+ What is the without regenerating NAD+, Oxidizing Agent of energyGlycolysis? production would stop! 9 H2O H2O Phosphoenolpyruvate (PEP) another molecule must accept HADP from NADH ATP so NAD+ is freed up for another round Phosphoenolpyruvate (PEP) 10 ADP ATP Pyruvate Pyruvate How is NADH recycled to NAD+? without oxygen with oxygen Another molecule aerobic respiration must accept the mighty electrons from pyruvate NADH NAD+ HO anaerobic respiration “fermentation” CO2 2 recycle NADH O2 NADH acetyl-CoA NADH NAD+ lactate What has more Free energy Pyruvate or Lactic acid? acetaldehyde NADH NAD+ lactic acid fermentation Krebs cycle ethanol alcohol fermentation Fermentation (anaerobic) Bacteria, yeast pyruvate ethanol + CO2 3C NADH 2C NAD+ beer, wine, bread 1C back to glycolysis Animals, some fungi pyruvate lactic acid 3C NADH 3C NAD+back to glycolysis cheese, anaerobic exercise (no O2) Pyruvate is a branching point Pyruvate O2 O2 fermentation anaerobic respiration mitochondria Krebs cycle aerobic respiration What’s the point? The point is to make ATP! ATP Oxidation of pyruvate Pyruvate enters mitochondrial matrix [ 2x pyruvate acetyl CoA + CO2 3C 2C 1C NAD Where does the CO2 go? Exhale! 3 step oxidation process releases 2 CO2 (count the carbons!) reduces 2 NAD 2 NADH (moves e ) produces 2 acetyl CoA Acetyl CoA enters Krebs cycle ] Krebs cycle 1937 | 1953 aka Citric Acid Cycle in mitochondrial matrix 8 step pathway each catalyzed by specific enzyme Hans Krebs 1900-1981 step-wise catabolism of 6C citrate molecule Evolved later than glycolysis does that make evolutionary sense? bacteria 3.5 billion years ago (glycolysis) free O2 2.7 billion years ago (photosynthesis) eukaryotes 1.5 billion years ago (aerobic respiration = organelles mitochondria) Count the carbons! pyruvate 3C 2C 6C 4C This happens twice for each glucose molecule 4C acetyl CoA citrate oxidation of sugars CO2 x2 4C 4C 6C 5C 4C CO2 Count the electron carriers! pyruvate 3C 6C 4C NADH This happens twice for each glucose molecule 2C 4C 4C acetyl CoA citrate reduction of electron carriers x2 FADH2 4C ATP CO2 NADH 6C CO2 NADH 5C 4C CO2 NADH Whassup? So we fully oxidized glucose C6H12O6 CO2 & ended up with 4 ATP! What’s the point? Electron Carriers = Hydrogen Carriers H+ Krebs cycle produces large quantities of electron carriers NADH FADH2 go to Electron Transport Chain! What’s so important about electron carriers? H+ H+ H+ + H+ H H+ H+ ADP + Pi ATP H+ Energy accounting of Krebs cycle 4 NAD + 1 FAD 4 NADH + 1 FADH2 2x pyruvate CO2 3C 3x 1C 1 ADP 1 ATP ATP Net gain = 2 ATP = 8 NADH + 2 FADH2 Value of Krebs cycle? If the yield is only 2 ATP then how was the Krebs cycle an adaptation? value of NADH & FADH2 electron carriers & H carriers reduced molecules move electrons reduced molecules move H+ ions to be used in the Electron Transport Chain like $$ in the bank What’s the point? The point is to make ATP! ATP Where is all of the energy after glycolysis and citric acid cycle? It is in the 4 ATP’s that were made But where is the rest of the energy? It is in the NADH and FADH2’s Time to break open the piggybank! Where can these Electrons go? So far the ATP’s have been generated via substrate level phosphorylation Now it’s time for chemiosmosis Where is the energy Coming from for the active transport? ??? What pulls electrons out of the chain? What happens to the energy that is lost from the electrons? AHH, the active transport! QuickTime™ and a decompressor are needed to see this picture. Peter Mitchell 1961 | 1978 Proposed chemiosmotic hypothesis revolutionary idea at the time Pyruvate from cytoplasm Inner + mitochondrial H membrane H+ Intermembrane space Electron transport C system Q NADH Acetyl-CoA 1. Electrons are harvested and carried to the transport system. NADH Krebs cycle e- e- FADH2 e- 2. Electrons provide energy to pump protons across the membrane. e- H2O 3. Oxygen joins with protons to form water. 1 O 2 +2 2H+ O2 H+ CO2 ATP Mitochondrial matrix H+ ATP ATP 4. Protons diffuse back in down their concentration gradient, driving the synthesis of ATP. H+ ATP synthase How are 34 ATPs made Variables in ATP yield: Some mitochondria differ in permeability to protons, which effects the proton motive force. Proton motive force may be directed to drive other cellular processes such as active transport. ATP yield is inflated by rounding up Prokaryotic cellular respiration is slightly higher since no mitochondrial membrane used to transport electrons from NADH. How do we use food other than glucose to generate energy?