Chemical Bond
advertisement

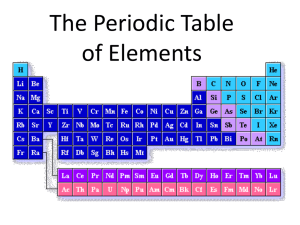
Bond Day 2: #4 Chemical Bond TEK I7D – relate the chemical behavior of an element including bonding, to its placement on the periodic table Day 2: #4 Before there were bonds, there had to be elements! Let’s review elements & the Periodic Table hide Periodic Table: Periods – horizontal rows Groups or Families – vertical columns Elements in the same group have similar chemical properties! Group Period Atomic number = # protons and it identifies the element!!!!! HI D E Valence Electrons – electrons involved in bonding; all atoms “want” to have 8 or 0 valence electrons to make them “happy and stable .” The numbers in purple are the # of valence electrons the atoms in those groups have. 1 8 HI 2 D E 3 4 5 6 7 Metals (pink elements) usually have 1, 2, or 3 valence electrons. In order to have 0 or 8 valence electrons, do you think a metal will lose or gain valence electrons? 1 8 HI 2 D E 3 4 5 6 7 Metals will lose electrons and become positive ions! Group 1 metals lose 1 ve- (which = 0 ve-) Group 2 metals lose 2 ve- (which = 0 ve-) Group 3 metals lose 3 ve- (which = 0 ve-) Remember to think about the easiest way to reach 0 or 8! 1 8 HI 3 4 5 6 7 2 D E When an atom loses or gains ve- it becomes an ion! Losing 1 ve- makes an ion with a +1 charge. Losing 2 ve- makes an ion with a +2 charge. Losing 3 ve- makes an ion with a +3 charge. 1 8 HI 2 D E 3 4 5 6 7 Nonmetals (yellow elements) usually have 5, 6, 7, or 8 valence electrons. In order to have 0 or 8 valence electrons, do you think a nonmetal will lose or gain valence electrons? 1 8 HI 2 D E 3 4 5 6 7 Nonmetals will gain electrons and become negative ions! Group 15 (5 ve-) nonmetals gain 3 ve- (which = 8 ve-) Group 16 (6 ve-) nonmetals gain 2 ve- (which = 8 ve-) Group 17 (7 ve-) nonmetals gain 1 ve- (which = 8 ve-) Remember to think about the easiest way to reach 0 or 8! 1 8 HI 3 4 5 6 7 2 D E When an atom loses or gains ve- it becomes an ion! Gaining 3 ve- makes an ion with a -3 charge. Gaining 2 ve- makes an ion with a -2 charge. Gaining 1 ve- makes an ion with a -1 charge. 1 8 HI 2 D E 3 4 5 6 7 What about Group 18 (the Noble Gases)? They already have 8 valence electrons so they are already “happy” and stable. They are the “perfect” elements and are considered unreactive! 1 8 HI 2 D E 3 4 5 6 7 What are Chemical Bonds? • An attraction between two or more atoms • Interaction between valence electrons • All atoms need 0 or 8 valence electrons to be “happy” or stable Two Kinds of Bonds • Ionic Bonds -form ionic compounds between metals and nonmetals -by losing /gaining electrons • Covalent Bonds -form covalent compounds -by sharing electrons 1. An unidentified element has many of the same physical and chemical properties as magnesium and strontium but has a lower atomic mass than either of these elements. What is the most likely identity of this element? F Sodium G Beryllium H Calcium J Rubidium Question from TEA released TAKS test Day 2: #4 2. The elements of which of these groups on the periodic table are most resistant to forming compounds? A Group 1 B Group 9 C Group 14 D Group 18 Question from TEA released TAKS test Day 2: #4 3. The elements from which of the following groups are most likely to react with potassium (K)? F Group 2 G Group 7 H Group 13 J Group 17 Question from TEA released TAKS test Day 2: #4 4. Which of the following groups contains members with similar chemical reactivity? A Li, Be, C B Be, Mg, Sr C Sc, Y, Zr D C, N, O Question from TEA released TAKS test Day 2: #4 5. According to the periodic table, which element most readily accepts electrons? A Fluorine B Nitrogen C Arsenic D Aluminum Question from TEA released TAKS test Day 2: #4 6. Elements in Group 16 of the periodic table usually — F form large molecules G gain electrons when bonding H act like metals J solidify at room temperature Question from TEA released TAKS test Day 2: #4