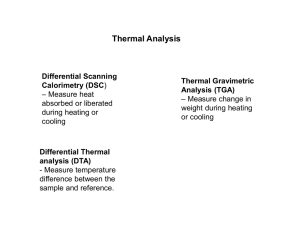
(DSC) Differential thermal analysis
advertisement

Materials Characterisation - Thermal Analysis • Introduction to thermal analysis • Information obtained. • Interpret & analyse data. Recommended Texts Textbooks • Differential Scanning Calorimetry: An introduction for practitioner, by G. Hohne, Springer (1996). • Differential Thermal Analysis: Fundamental aspects, Vol 1., edited by R.C. Mackenzie, Academic Press (1970). • Differential Thermal Analysis: Applications, Vol. 2, edited by R.C. Mackenzie, Academic Press (1970). •Differential Thermal Analysis: A guide to techniques and its applications, by M. I. Pope, Heyne (1977). Journal papers • “Differential Scanning Calorimetry: applications in drug development”, by S-D Clas, C.R. Dalton, B.C. Hancock, PSTT, Vol 2(8), pp311-320 (1999). • “DSC analyses of the precipitation behaviour of two Al-Mg-Si alloys naturally aged for different times”, by L. Zhen and S.B. Kang, Materials Letters, Vol 37, pp349-353 (1998). Introduction to thermal analysis Purpose: to provide quantitative information about exothermic, endothermic and heat capacity changes as a function of temperature and time. Types of thermal analysis techniques: Differential scanning calorimetry (DSC) Differential thermal analysis (DTA) Thermogravimetry (TG) Thermomechanical analysis (TMA) Definitions • A calorimeter measures the heat into or out of a sample. • A differential calorimeter measures the heat of a sample relative to a reference. • A differential scanning calorimeter does all of the above and heats the sample with a linear temperature ramp. • Endothermic heat flows into the sample. • Exothermic heat flows out of the sample. DSC: The Technique • Differential Scanning Calorimetry (DSC) measures the temperatures and heat flows associated with transitions in materials as a function of time and temperature in a controlled atmosphere. • These measurements provide quantitative and qualitative information about physical and chemical changes that involve endothermic or exothermic processes, or changes in heat capacity. Conventional DSC Empty Sample Metal 1 Sample Temperature Metal Metal 2 1 Metal 2 Reference Temperature Temperature Difference = Heat Flow Netsch DSC 404 F1 Pegasus •A “linear” heating profile even for isothermal methods Technical Group Talk Modes and principles of operation (1) (b) Power compensated DSC: Temperature differences between the sample and reference are ‘compensated’ for by varying the heat required to keep both pans at the same temperature. The energy difference is plotted as a function of sample temperature. Modes and principles of operation (2) (c) Heat flux DSC ultilizes a single furnace. Heat flow into both sample and reference material via an electrically heated constantan thermoelectric disk and is proportional to the difference in output of the two themocouple junctions. Modes and principles of operation (3) (a) DTA: difference in temperature between the sample and reference is plotted against sample temperature. Influence of Sample Mass 0 DSC Heat Flow (W/g) Indium at 10°C/minute Normalized Data -2 Onset not influenced by mass 15mg 10mg 4.0mg -4 1.7mg 1.0mg 0.6mg -6 150 6 152 154 156 158 160 Temperature (°C) 162 164 166 Effect of Heating Rate on Indium Melting Temperature 1 Heat Flow (W/g) 0 -1 -2 heating rates = 2, 5, 10, 20°C/min -3 -4 -5 154 6 156 158 160 162 164 Temperature (°C) 166 168 170 The temperature difference (DT) between the reference cell (Trm) and the sample cell (Tsm) in DSC can be expressed as: DT = Trm – Tsm = R(dT/dt) (Cs – Cr) Where R = thermal resistance dT/dt = heating rate Cs = heat capacity of sample Cr = heat capacity of reference A change in the heating rate (dT/dt) results in a shift in DT corresponding to the observed hysteresis shifts in the DSC trace DSC: Main Sources of Errors •Calibration •Contamination •Sample preparation – how sample is loaded into a pan •Residual solvents and moisture. •Thermal lag •Heating/Cooling rates •Sample mass •Processing errors Sample preparation Form of sample: bulk solid, powder (pressed), liquid. Amount of sample: 3-5mg. DSC Pan: Al, Pt, stainless steel, Ag, Cu, Al2O3 Sample Preparation : Shape • Keep sample as thin as possible (to minimise thermal gradients) • Cover as much of the pan bottom as possible • Samples should be cut rather than crushed to obtain a thin sample (better and more uniform thermal contact with pan) 99 Operation procedures • Calibration of instrument • temperature, heat of reaction, heat capacity scale using high purity standards (In, Sn, Bi, Pb,Au) • Baseline correction for a given scan rate (1 - 40K/min). • Weight samples before (and maybe after) experiment. Beware of any contamination or reaction with specimen pans. Enthalpic transition studied by DSC or DTA Endothermic: Fusion, vaporization, sublimation, desorption, reduction, decomposition, degradation. Glass transition (e.g. baseline shift). Exothermic: Crystallization, condensation, solidification, adsorption, precipitation, oxidation, degradation, curing of resins. Type of DSC experiments Dynamic heating - thermodynamic properties Isothermal heating - kinetic parameters What can DSC/DTA measure? •Glass transitions •Melting and boiling points •Crystallisation time and temperature •Percent crystallinity •Heats of fusion and reactions •Specific heat capacity •Oxidative/thermal stability •Rate and degree of cure •Reaction kinetics •Purity Dynamic heating Constant heat rate mode. (e.g. heat flow vs. temperature). What can we characterise? Heat Flow -> exothermic DSC Thermogram Cross-Linking (Cure) Crystallisation Glass Transition Melting Temperature 6 Oxidation Polymer Glass transition, crystallisation and melting Endo Glass transition Crystallization Melting Example DSC - PET Sample : PET80PC20_MM1 1min Size : 23.4300 mg Method: standard dsc heat -cool-heat Comment : 5/4/06 DSC File: C:...\DSC\Melt Mixed1\PET80PC20_MM1.001 Operator : SAC Run Date : 05-Apr-2006 15 :34 Instrument : DSC Q1000 V9.4 Build 287 Tm 1.5 245.24°C Tc 1.0 Heat Flow (W/g) Tg 137.58°C 20.30J/g 79.70°C(I) 0.5 228.80°C 22.48J/g 81.80°C 75.41°C Cycle 1 144.72°C 0.0 -0.5 0 Exo Down 50 100 150 Temperature (°C) 200 250 300 Universal V4.2E TA Instruments Polymer Glass transition temperature, Tg. Endothermic • Tg is characterized by a change in heat capacity, (i.e. a change in the baseline). • Relaxation process is quantified as the volume relaxation or enthalpy relaxation. • It is characterized in DSC by an endothermic peak or enthalpy overshoot at the Tg. • It is highly dependent on the thermal history of the sample. Solid to liquid transformation: Melting or solidification endothermic Purity Ts Te 2 RTe X DH 0 Fi Where Ts =sample temp Te = melting temp of pure component R = gas constant X =molar fraction of impurity DHo= enthalpy of fusion at Ts F = fraction of sample molten. Determination of fraction transformed, x(t) Peak onset x= subtended area complete area Temp Fig.1. Schematic diagram of a DSC peak, showing the subtended area and the evaluation of x for a particular Temp time. endothermic Heat flow Peak end Metal: Au alloys Solid to liquid transformation: Melting or solidification DTA of Au-Ag-Cu-Zn alloy 1.4 Endo Heat Flow (W/g) 1.2 1 0.8 0.6 0.4 0.2 0 700 Heating Cooling 750 800 850 Temperature (C) 900 950 1000 Solid-state transformation Precipitation in Al alloys in (A) Al-095Mg-0.85Si-0.3Mn-0.1Zr (wt%) alloys Peaks 1and 3a: clusters of Si and Mg atoms and small ppt formation. Peak 2: Guinier and Preston (GP) zones formation. Peaks 3b and 4: Precipitation of b” and b’ phases Peaks 5 and 6: Formation of Si precipitates and b phase. Solid-state transformation: Oxidation. 5 32m Endothermic Heat flow (mW/mg) 0 -5 70nm -10 -15 100nm 77nm Exothermic -20 500 550 600 Temperature(C) 650 700 Solid State Transformation Crystallisation from amorphous phase Exothermic Amorphous (glassy) Crystallisation (Devitrification) Crystalline (LRO) Tx increases with increasing Y/Ni ratio. Tx increases with Cu addition. Analysis of DSC/DTA data From dynamic heating mode. Information: 1. Transformation temperature (e.g. onset, peak). 2. Transformation enthalpy (e.g. area under the peak). 3. Activation energy for transformation (e.g. Kissinger analysis) Kissinger analysis Tp 2 E a C ln b RTp Where Tp = peak temperature b = heat rate Ea = activation energy R = gas constant (8.314 J/K mole) C = constant Plot ln ( Tp2 / b ) vs 1/Tp , gradient = Ea / R. Kissinger analysis DSC traces of Ni50.54Ti49.46 thin films at different heating rates. Plot of the Kissinger equation for the crystallization in Ni50.54Ti49.46 thin films. The activation energy of amorphous Ni50.54Ti49.46 thin films was 411 kJ/mol, Isothermal heating mode Heating at a fixed temperature over a time interval. (e.g. heat flow vs. time) exothermic Isothermal heating Onset Dynamic heating of amorphous melt spun Fe63Cr18Ti4B15 ribbons End Isothermal heating of amorphous melt spun Fe63Cr18Ti4B15 ribbons Determination of fraction transformed, x(t) Peak onset x= subtended area complete area Time Fig.1. Schematic diagram of a DSC peak, showing the subtended area and the evaluation of x for a particular time. exothermic Heat flow Peak end Transformation Kinetics The characteristic of the kinetics (e.g. fraction transformed versus time plot) is that of the “S-curve”, i.e. slow at first, then accelerating, then decelerating. Johnson-Mehl-Avrami Equation n x ( t ) 1 exp( kt ) Where x(t) = volume fraction transformed at time t n = Avrami exponent , which is dependent on nucleation rate and growth rate. k = rate constant. Johnson-Mehl-Avrami Analysis n x ( t ) 1 exp( kt ) Rewrite in ln form: ln(-ln(1-x)) = ln k +nln(t) Plot ln(-ln(1-x)) vs ln(t) • Straight line with a gradient n. • Intercept is ln k Arrhenius equation Rate constant, k is dependent on temperature according to the Arrhenius equation given by: k = A exp (-Ea / RT) where A = pre- exponential factor, Ea = activation energy, R = gas constant and T = temperature (K) Re-write in ln form, gives: Ln k = ln A - Ea/RT. Plotting ln k vs 1/T • Straight line with a slope of Ea/R • Determine activation energy Ea. Summary • Operating principle of DSC, DTA. • Dynamic and Isothermal heating mode. • Interpretation of data to extract information such as transition temperature, activation energy, kinetics etc.