1. dia
advertisement

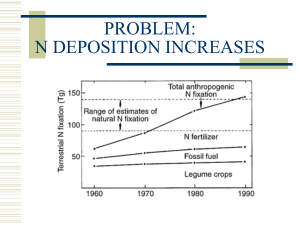
BUDAPEST FACULTY UNIVERSITY OF TECHNOLOGY AND ECONOMICS OF CHEMICAL AND BIOCHEMICAL ENGINEERING DEPARTMENT OF CHEMICAL AND PROCESS ENVIRONMENTAL ENGINEERING NITROGEN-OXIDES Authors: Dr. Bajnóczy Gábor Kiss Bernadett The pictures and drawings of this presentation can be used only for education ! Any commercial use is prohibited ! Nitrogen oxides In the atmosphere: NO, NO2, NO3, N2O, N2O3, N2O4, N2O5 Continuously : only NO, NO2, N2O The others decay very quickly : Into one of three oxides Reaction with water molecule NO nitric oxide colourless odourless toxic nonflammable NO2 nitrogen dioxide reddish brown strong choking odour very toxic nonflammable N2O nitrous oxide colourless sweet odour non-toxic nonflammable Physical properties of NO, NO2 and N2O Nitric oxide NO Molecular mass Melting oC point Boiling oC point density 0 0 C, 101.3 kPa 25 0C, 101.3 kPa Solubility in water 0 0 C 101.3 kPa Conversion factors 0 0C, 101.3 kPa Nitrogendioxide NO2 Nitrous oxide N2O 30 46 44 -164 -11 -91 -152 21 -89 1.250 g/dm3 1.145 g/dm3 73,4 cm3/ dm3 (97.7 ppmm)** 1 mg/m3 = 0.747 ppmv*** 1 ppmv = 1.339 mg/m3 2.052 g/dm3 1,916 g/dm3 1,963 g/dm3 1.833 g/dm3 bomlik decay 1305 cm3 /dm3 • NO2 under 0ºC colourless nitrogen tetroxide (N2O4) •NO2 natural background – 9,4 μg/Nm3 (0,2 – 5 ppb) • in urban area : 20 – 90 μg/Nm3 (0,01 – 0,05 ppm) • sometimes : 240 – 850 μg/Nm3 (0,13 – 0,45 ppm) • N2O background ~ 320 ppb 1 mg/m3 = 0.487 ppmv*** 1 ppmv = 2,053 mg/m3 1 mg/m3 = 0,509 ppmv*** 1 ppmv = 1,964 mg/m3 0,4 Nitrogen oxides Environment: NO and NO2 acidic rain, photochemical smog, ozone layer destroyer N2O : stable No photochemical reactions in the troposphere ► lifetime 120 year Natural background : 313 ppmv Rate of increase 0,5-0,9 ppmv/year Greenhouse effect showed itself recently Natural sources of nitrogen oxides Atmospheric origin of NO: Electrical activity (lightning) 20 ppb NO HNO3 transition → continuous sink Equilibrium concentration is kept by the biosphere: see: nitrogen cycle ~ Nitrogen-oxides (NO, N2O) from bacterial activity • NO emission by the soils 5-20 μg nitrogen/m2 hour, function of organic and water content and temperature • Natural N2O : oceans, rivers Natural sources of nitrogen oxides Electrical activity in the atmosphere; lightning N2 + O2 => 2 NO Organic nitrogen content of the soil is decomposed by micro organisms Bottom of the river, anaerobic condition, microbiological activity Anthropogenic sources of nitrogen oxides Transportation Fuel combustion Application of nitrogen fertilizers Anthropogenic sources of nitrogen oxides NO: Fossils fuel combustion: power plants and transportation Agriculture: Nitrogen fertilizers increase the microbiological activity resulting in NO emission N2O: Agriculture: Nitrogen fertilizers increase the microbiological activity resulting in N2O emission Transportation (three way catalyst system) Power plants (fluid bed boilers) Chemical industry (nitric acid) 0,2 % yearly increase in atmospheric content. Formation of nitric oxide: Thermal way • N2 : strong bond in the molecule → no direct chemical reaction with oxygen Chain reaction: (Zeldovich, 1940) O forms in the flame N2 + O = NO + N N + O2 = NO + O N + •OH = NO + H → rate limiting step The concentration of atomic oxygen is the function of the flame temperature. ▼ thermal way dominates above 1400 ºC Rate limiting factors of thermal NO Temperature [ 0C ] NO concentration at equilibrium [ ppm ] Time 500 ppm [ sec ] 27 1,1 x 10 -19 - 527 0,77 - 1316 550 1370 1538 1380 162 1760 2600 1,1 1980 4150 0,117 The amount of thermal NO is the function of the flame temperature and the residence time Formation of prompt NO Fenimore, 1970: low flame temperature Hydrocarbons ▬▬▬▬▬▬▬▬▬► 1000 oC The reactions starts by the alkyl radicals. • CH + • CH2 + • CH3 + • • + N2 = HCN + N → rate determination step • CH + N • 2 2 = HCN + NH • CH + N • 3 2 = HCN + NH2 • CH High temperature flame section: HCN + O = NO + • CH • NH + O = NO + H • NH + • OH = NO + H 2 The prompt NO is slightly temperature dependent (approx: 5% of the total). NO from the nitrogen content of the fuel • The bond energy of C-N in organic molecule : (150 – 750 kJ/mol), smaller …than N-N in the nitrogen molecule → increased reactivity • not sensitive to the flame temperature, • sensitive to the air excess ratio • in oxygen lean area (reduction zone) the HCN and NH3 are reduced to …nitrogen NO2 formation in the flame Only a few % of NO2 can be found in the stack gas NO2 starts to decompose above 150 °C and total decay: above 620 °C At low flame temperature: NO + •HO2 = NO2 + • OH Formation of hydroperoxyl radicals: At high flame temperature: H + O2 + M = • HO2 + M H + O2 = • OH + O Significant part of NO2 returns back to the higher flame temperature section : • decays thermally NO2 = NO + O • chemical reaction transforms back to NO: NO2 + H = NO + • OH NO2 + O = NO + O2 Formation of N2O : Low temperature combustion ~10-50% of the fuel N at 800 ºC – 900 ºC may transform to N2O. In exhaust gas → 50 – 150 ppmv N2O Thermal decay of coal → hydrogen cyanide formation HCN + O = NCO + H NCO + NO = N2O + CO There is no N2O above 950 ºC , decays thermally above 900 ºC N2O + M = N2 + O+ M Increasing temperature favours the formation of hydrogen atoms → reduction N2O + H = N2 + •OH Fuels with low heat value (biomass) favours the formation of N2O N2O formation by catalytic side reactions • • Anthropogenic N2O source : automobiles equipped with catalytic converter By products of three way catalytic converters: 1. NO reduction 2. CO oxidation 3. Oxidation of hydrocarbons temperature product of side reaction increase suppresses the reaction Adsorption, dissociation On the surface of catalyst product of main reaction N2O emission from automobiles Catalyst type mg/km year without ~ 10 1966 - 1972 Installation of catalysts increases the N2O emission. Two way system (oxidation) ~27 1978 - 1982 The benefit > the drawback ~46 1983 - 1995 ~19 1996 - Three way system (oxidation – reduction) Three way system (oxidation – reduction) Diesel engine ~ 10 Summary of the nitrogen oxide formation in the flame Simplified reaction way Above 1400 0C, strongly temperature dependent, forms in the oxidation zone Thermal NO Above 1000 0C, slightly temperature dependent, forms in the reduction zone Prompt NO NO from the fuel Thermal decay Organic-N Above 1000 0C, slightly temperature dependent, forms in the oxidation zone. Forms in the cooler part of the flame, decays in warmer parts NO2 N2O remark Thermal decay Organic-N Forms in the range of 800 0C – 900 0C, decays at higher temperatures NO → NO2 transformations in the troposphere Possible reaction with O2 → slow Formation of hydroxyl radicals NO oxidation by hydroxyl radicals NO oxidation by methylperoxy radicals The pure cycle of NO in the troposphere The ozone molecule may react with another molecule N2O in the atmosphere Source: natural and anthropogenic Very stable in the troposphere: No reaction with the hydroxyl radicals λ >260 nm → there is no absorption Previously it was not considered polluting material. Recently came to light: greenhouse effect gas Fate of nitrogen oxides from the atmosphere Nitric oxide, nitrogen dioxide • NO photochemically inert, no solubility in water, forms to NO2 • NO2 soluble in water: Another way of NO2 elimination: NO2 + H2O → HNO3 + HNO2 NO2 + O = •NO3 •NO3 + NO2 = N2O5 N2O5+ H2O = 2 HNO3 ▼ Effect of light slow Only after sunset. Nitrous oxide N2O Transport from the troposphere to the stratosphere, here decays: • oxidation: •photochemical decay: N2O + O = 2 NO Detrimental effect: decays the ozone layer: 260 nm N2O N2 + O The human activity continuously increases the N2O concentration of the atmosphere. There is a 0,25% increase /year Effect of nitrogen oxides on Plants Outspokenly harmful In the atmosphere NO and NO2 together (NOx) 10 000 ppmv NO → reversible decrease of photosynthesis NO2 → destruction of leaves (formation of nitric acid), cell damages Effect of nitrogen oxides on Humans NO2 is four times toxic than NO Odor threshold: 1-3 ppmv Mucos irritation: 10 ppmv 200 ppmv 1 minute inhaling → death! Origin of death: wet lung Nitric acid formation in the alveoli Alveoli have semi permeable membrane (only gas exchange is possible) Nitric acid : destroys the protein structure of the membrane → the alveoli is filled up by liquid No more free surface for the gas exchange → death Effect of nitrogen oxides on constructing materials Acid rain causes electrochemical corrosion Surface degradation on limestone, marble by the acidic rain. Control of nitrogen oxides emission Technological developments: only 15% decrease (since 1980) ~90% of anthropogenic emission comes from boilers internal combustion engines Control of emission: make conditions do not favor the formation elimination of the nitrogen oxides from the exhaust gases Control of nitrogen oxides emission The NO formation in the flame depends on: N content of the fuel Flame temperature Residence time in the flame Amount of reductive species The air excess ratio (n) has strong effect on the last three. The air excess ratio can be adjusted globally or locally. Control of nitric oxide (NO) emission, by two stage combustion Two stage combustion: the air input is shared to create different zones in the flame → a./ reduction zone where the combustion starts b./ oxidation zone where the combustion is completed. oxidation zone secondary air fuel + air secondary air reduction zone Control of nitric oxide (NO) emission by two stage combustion BOILER Control of nitric oxide (NO) emission, by three stage combustion ZONES IN THE FLAME: 1. Perfect burning in the most inner part of the flame (oxidation zone). 2. Fuel input to reduce the NO (reduction zone). 3. Finally air input to oxidize the rest of hydrocarbons (oxidation zone). burner Control of nitric oxide (NO) emission by three stage combustion Control of nitric oxide (NO) emission, by three stage combustion 1. zone fuel (coal powder, oil) ( n>1) 2. zone 10..20% fuel input n=0,9 temperature 1000°C 3. zone air input, n>1, perfect burning. 30..70% NO reduction is available Flue gas recirculation Application: oil and gas boilers The cooled flue gas has high specific heat due to the water content. The recirculated flue gas decrease the flame temperature. Generally ~10% is recirculated More than 20 % produces higher CO and hydrocarbon emissions. 1. Mixed with air input (FGR: flue gas recirculation) 2. Mixed with fuel input (FIR: fuel induced recirculation) Nitric oxide (NO) eliminations from the exhaust gas possibilities: Selective noncatalytic reduction SNCR (thermal DENOx process) Selective catalytic reduction SCR (catalytic DENOx process) Reduction of NO emission by selective non catalytic reduction Ammonia is added to the NO contaminated fuel gas at 900 ºC: 4 NO + 4 NH3 + O2 = 4 N2 + 6 H2O Danger of excess ammonia. Better solution is the urea 2 NH2▬CO▬NH2 + 4 NO + O2 = 4 N2 + 4 H2O + 2 CO2 • advantage: simplicity • disadvantage: temperature sensitive. • ammonia: 870 – 980 ºC, urea 980 – 1140 ºC At higher temperature ammonia is oxidized to NO At lower temperature ammonia remains in the fuel gas Efficiency : 40 – 70 % at optimal condition. Reduction of NO emission by selective catalytic reduction • better efficiency is available • composition: V2O5 or WO3 on titanium dioxide supporter • Applied NH3 / NO rate ~0,8 (mol/mol), Drawback: • SO2 content of the fuel gas is oxidized to SO3 → corrosion • Ammonium-sulphate deposition on the catalyst surface •The method can not be applied over 0,75 % sulfur content in the stack gas NO elimination from the exhaust gas of internal combustion engines Only the treatment of the exhaust gas is possible Control methods applied to one pollutant often influence the output of other pollutant NO elimination from the exhaust gas of internal combustion engines NO from internal combustion engine is thermal origin. NO elimination by selective catalytic reduction. Discussed in details at hydrocarbons