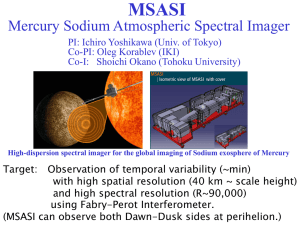
Parameter Analysis of a Practical Lithium
advertisement

Advanced Power Sources for EVs E. Peled School of Chemistry Tel Aviv University, Tel Aviv, Israel IFCBC 26.1.2011 http://www.tau.ac.il/institutes/ifcbc/presentations-2010.html 1 Issues • Introduction • Comparison between fuel cells and lithium ion power sources for EVs • Advantages and limitations of lithium air battery (recently attracting a lot of attention) • Advantages and limitations of a novel sodium air battery • Preliminary performance of sodium air battery • Summary 2 Product: Li2O Li2O2 Without oxygen 3 Source - IBM 2010 4 Peter Bruce 2010 Na – air Li -air 5 Disadvantages of the lithium–air cell • Very low power* - about 0.1 to 1mA/cm2 mainly due to a sluggish oxygen-reduction reaction (ORR). • The oxygen-discharge product is lithium peroxide (a very strong oxidizing agent) which is very reactive toward the electrolyte solvents and the environment. • In addition, it is an electrical insulator, thus a large area of carbon substrate is required to accommodate the solid peroxide at a thickness lower than the tunneling range of electrons (about 2nm). • Sensitive to water and CO2 penetration. • Safety issues, especially due to lithium dendrite formation. 6* * K. Abraham, P. Bruce, S. Mukerjee Dendrite Formation on Charge • In all nonaqueous lithium batteries, the anode is covered by a thin film called a Solid Electrolyte Interphase (SEI)*. • As a result, on charge, lithium deposits through the SEI in the form of lithium dendrites and mossy (sponge) lithium. • This raises safety issues – the formation of internal short circuits by lithium dendrites. • For these reasons, efforts to develop rechargeable lithiummetal batteries have failed and today only rechargeable lithium-ion batteries, which do not contain metallic lithium, are in use. * E. Peled The Electrochemical Behavior of Alkali and Alkaline Earth Metals in Nonaqueous Battery Systems -The Solid Electrolyte Interphase (SEI) Model. J. Electrochem. Soc. 126, 7 2047-2051 (1979). Charge Preliminary evaluation of energy- and power-density constraints for a lithium – air battery (for a bipolar-plate battery design) • Assuming a 100 liter, 100kWh, 100kW stack, we obtain 1W/ml and 1Wh/ml. • Assuming 2mm-thick cells (Vs. 0.2 mm in Li ion batteries), at 2.5V, the current and charge densities are 80mA/cm2 and 80mAh/cm2. • Assuming 1Ah/g of carbon, we obtain 80mg carbon/cm2 and about 0.8mm-thick empty carbon electrode. • For a 50 liter stack (a volume similar to that of the Honda FCX Clarity FC stack (57 liter), we get 160mA/cm2 and 160mAh/cm2, and about 1.6mm-thick empty carbon electrode. • Conclusion: Due to a thick air electrode, the lithium-air battery, having the BPP design, has to run at about 0.1A/cm2 in order to have a practical volume and weight. 8 Molten sodium–air nonaqueous battery • We suggest here a novel concept, namely to replace the metallic lithium anode by liquid sodium (absorbed in a porous matrix) and to operate the sodium–air (oxygen) cell above the sodium melting point (97.8oC). • The theoretical specific energy of the sodium–air cell, assuming Na2O as the discharge product and including the weight of oxygen, is • 1690 Wh/kg, about four times that of state-of-the-art lithium-ion batteries. • (The average specific energy density of the Na/O2 cell is 1980Wh/kg) 9 Advantages of molten sodium as an anode for a rechargeable air cell • Sodium is much cheaper and more abundant than lithium. • The surface tension of the liquid sodium anode is expected to prevent the formation of sodium dendrites on charge. Any sodium dendrites that might be formed would be absorbed into the liquid phase. • The higher operating temperature accelerates electrode kinetics and reduces electrolyte resistance, thus enabling running the cell at higher power. • Sodium peroxide is less stable and more reactive than lithium peroxide and can be decomposed by a manganese dioxide. 10 Advantages of molten sodium as an anode for a rechargeable air cell (cont.) • At the higher operating temperature and with the use of a proper fourelectron ORR catalyst, it may be possible to reversibly reduce oxygen to oxide (as Na2O), thus avoiding the accumulation of peroxide in the air electrode. • In contrast to lithium, sodium does not dissolve in aluminum (0.003%) and this enables the use of thin aluminum foil as a light and low-cost hardware material, especially for thin bipolar plates. • By contrast, lithium cells require the use of copper or nickel as anode current-collector materials, both of them heavier and much more expensive than aluminum. • At temperatures above 100oC, little if any interference of atmospheric water is expected. • In addition, unlike lithium, sodium does not form a nitride in air. • The adsorption of CO2 may be reversed by the oxidation of sodium carbonate to oxygen and CO2 on charge. 11 The disadvantages of the sodium-air battery (in comparison to the room-temperature lithium-air battery) • The open-circuit voltage of the sodium-oxygen cell is 2.3-2.4V, lower than that for the lithium– oxygen cell (3V). • It has a lower specific energy. • At present, the cycling (coulombic) efficiency of molten sodium, covered by an SEI at 110oC (70 -90%) is not high enough, and increasing it to nearly 100% presents a challenge (it is low for a fresh cell and rises to over 95% during cycling) . • At present, SEI resistance is too high (about 200 Ohm.cm2). 12 Sodium SEI Issues • In order to create a protective SEI on alkali metal anodes it is essential that the equivalent volumes of the SEI materials be larger than that of the anode*. • Only in this way, the SEI can completely cover the anode surface and stop corrosion. If not, the anode will continue to corrode. • The equivalent volumes of Na2CO3, NaF and Na2O are lower than that of sodium.Thus these cannot serve as good SEI-building materials. • On the other hand, the equivalent volumes of several sodium oxosulfur materials including: Na2S2O4, Na2S2O3 are larger than that of sodium, thus they are suitable candidates for use as sodium SEI-building materials. • * E. Peled, D. Golodnitsky, C. Menachem, and D. Bar Tow. An Advanced Tool for the Selection of Electrolyte Components for Rechargeable Lithium Batteries J. Electrochem. Soc., Vol. 145, No. 10, October 1998 13 Sodium - Air Battery – FC BPP Stack Design Cell thickness (including a cooling cell) is estimated to be about 2 mm 14 Molten sodium–air cells, preliminary results at above 100oC* *Parameter analysis of a practical lithium-and sodium-air electric vehicle battery E. Peled, D. Golodnitsky, H. Mazor, M.Goor, S. Avshalomova; Journal of Power Sources xxx (2010) xxx–xxx 15 -4 1.0x10 -4 3.5 3.0 charge 2.5 5.0x10 -5 2.0 discharge 1.5 0.0 1.0 -5.0x10 -1.0x10 Voltage (V) Current (A) 1.5x10 -5 V I 0.5 -4 2000 3000 4000 5000 0.0 6000 Test Time (s) Discharge/charge curves of a Na-O2 cell at 105oC Voltage range of 1.5V-3.0V (or 20 minutes operation time), discharge and charge currents are 50µA and 100µA respectively, (FC hardware, electrode area – 1cm2, 16 ETEK cathode): The electrolyte is based on PEGDME 2000 and PC. -4 1.0x10 -4 Oxygen Starvation- Voltage/Current Profile 3.5 3.0 2.5 5.0x10 -5 2.0 1.5 0.0 1.0 -5.0x10 -1.0x10 Voltage (V) Current (A) 1.5x10 -5 V I -4 6000 0.5 O2 flow off 7000 8000 O2 flow on 9000 0.0 10000 Test Time (s) Oxygen starvation of a Na-O2 cell at 105oC Voltage limits 1.5 - 3V and 1 min rest at OCV, charge and discharge at 100µA and 50µA, respectively. (FC hardware, electrode area – 1cm2, ETEK cathode): The 17 electrolyte is based on PEGDME 2000 and 10%PC. Charge discharge cycles of sodium – air cell at 110oC Ch. at 100μA/cm2, Dis. at 40μA/cm2, Voltage limit 1-4V, time limit 0.5h (PE based) The problem: A rise of the charging voltage with cycle number. Sodium plating and dissolution at above 100oC • In order to prove that sodium can be cycled in its molten state, we ran deposition–dissolution tests of sodium on aluminum at 110oC (above the melting point of sodium). • We added methyl methanesulfonate as an SEI precursor and obtained, after some SEI building cycles, cycling current efficiency of 70 to 90%. 19 Sodium cycling efficiency at 1100C (Na/SS cell, time range = 11350min - 12000min) =70 - 90% No dendrites formation During over 300 hours and over 400 cycles! Nonaqueous Alkali Metal-Air EV Battery - Summary Issues that need to be addressed: • • • • • Power must be increased by two orders of magnitude (up to about 0.1 A/cm2 following the use of thick cells, (Rcell = 1 to 10 Ohm.cm2). Peroxide formation must be avoided (obtain a reversible 4e ORR). Dendrite formation on charge must be avoided. Sensitivity to moist air and CO2 should be reduced, or use an efficient water barrier. The battery should be preferably assembled in the discharged state (by charging the cathode with carbonate). The use of a liquid sodium anode at above 100oC may solve or ease these problems: • • • • • • • 21 Accelerates sluggish cathode reactions and lowers cell impedance. Dendrites are not formed. It is easier to obtain a reversible 4e ORR and avoid peroxide formation. Interference by water vapor and CO2 is minimized. We found indications for carbonate decomposition on the first charge and this may enable battery assembly in the discharge state. In addition - lighter and lower-cost hardware material (aluminum) is used. Preliminary results show: (a) the functioning of the molten sodium-air battery; (b) high faradaic efficiency of the sodium plating–dissolution process and (c) possibility of oxidizing sodium carbonate. Conclusions • In the near future all-electric battery-powered electric vehicles will find niche applications as city cars and limited range commuter cars. • Lithium and sodium – air batteries can make a major breakthrough in battery technology and cost giving Evs, in the long term, a driving range of 500 km. • The fuel cell electric vehicle could provide the range and refueling times at an affordable price demanded by modern drivers for full function passenger vehicles. • Market penetration will follow the order: HEV < PHEV < FCEV < BEV 22 Secretary Chu's (US Secretery of Energy) addressed the United Nations Climate Change Conference in Cancun (December 2010). • "A rechargeable battery that can last for 5,000 deep discharges, 6-7 x higher storage capacity (1,000 Wh/Kg) at 3x lower price will be competitive with internal combustion engines (400 - 500 mile range).“ • The only battery chemistries that have a chance of achieving energy densities in the 1,000 wh/kg range are rechargeable metal-air Thank you for your attention Acknowledgments Prof. D. Golodnitsky, H. Mazur, M. Goor and S. Avshalomi 24 Na deposition 50A 0.06 1.0 0.04 0.5 0.0 0.02 -0.5 0.00 -0.02 615 I 630 -1.0 25A o Na dissolution V 645 660 675 690 Potential [V] Current [mA] 1.5 o -1.5 705 time [min] Sodium deposition-dissolution on Al at 105oC. Na/NaTf:PEO6 + Methyl methanesulfonate 5%(wt)/Al cell. Discharge 25 and charge rates are 50µA for 10 min and 25µA for 20 min, respectively. Coin-cell hardware. Electrode area - 0.57cm2. Capacity [mAh] 0.015 o dissolution of Na o deposition of Na 0.010 0.005 discharge=50A, 10min charge=25A, 20min 0.000 0 20 40 60 80 100 120 140 Cycle No. Deposition and dissolution cycles of sodium on Al at 105oC Na/NaTf:PEO6 + Methyl methanesulfonate 5%(wt)/Al coin cell. Discharge and charge rates are 50µA for 10 min and 25µA for 20 min, respectively. 26 -700 SEI apparent thickness of 94Å is attributed to the AC after discharge second semi-circle of 1.7µF capacitance and 330 Å to the first one. -600 Z''/ohm -500 Rbulk=60 -400 RSEI,2=620 Capacity =1.7F RSEI,1=540 Capacity=0.2F -300 -200 -100 0 0 400 800 1200 1600 2000 Z'/ohm AC impedance spectra of Na/NaTf:PEO6 + Methyl methanesulfonate 5%(wt)/Al cell 105oC. 27 After plating of Na on Al, frequency range - 10MHz to 1mHz. Electrode area - 0.57cm2 . 28 29 PEM FC stack 30 31 Honda FCX Clarity FC Stack 1.75 kW/l 1.5 kW/kg