PHOSPHORUS CYCLE
advertisement
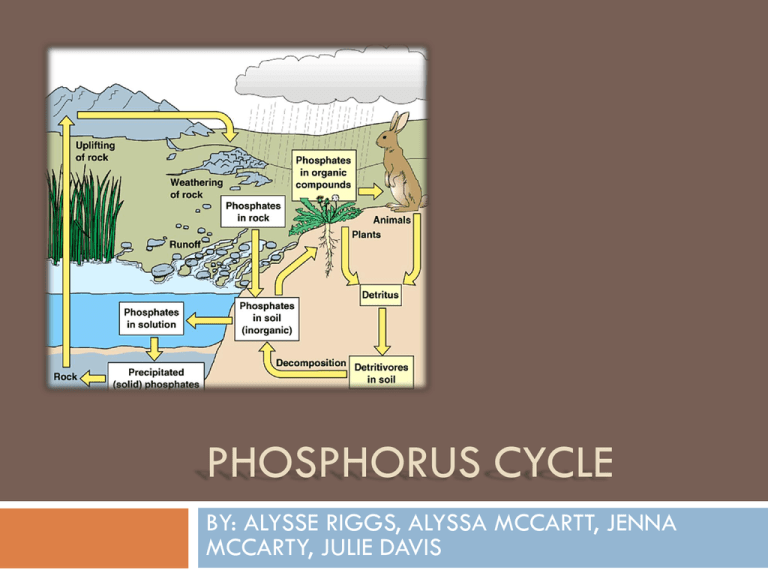
PHOSPHORUS CYCLE BY: ALYSSE RIGGS, ALYSSA MCCARTT, JENNA MCCARTY, JULIE DAVIS The Chemical Reactions Involved Reaction of phosphorus with air: White phosphorus glows in the dark when exposed to damp air in a process known as chemuluminescence. White phosphorus must be handled with great care. It spontaneously ignites in air at about room tetraphosphorus decaoxide. P4 (s)+502 (g) P4010(s) Reactions Cont. Reaction of phosphorus with the halogens: white phosphorus, P4, reacts vigorously with all the halogens at room temperature to form phosphorus trihalides. So, it reacts with fluorine, chlorine, bromine, and iodine, to form respectively phosphorus (III) fluoride, phosphorus (III) chloride, phosphorus (III) bromide, and phosphorus (III) iodide. P4(s) + 6F2(g) P4(s) + 6Cl2(g) P4(s) + 6Br2(g) P4(s) + 6I2(g) 4PF3(g) 4PCl3(I) 4PBr3(I) 4PI3(g) Reactions Cont. The reactions involving Phosphorus are: Substitution reactions: one group on phosphorus is replaced by another group; has two possiblities. Oxidation reactions: oxidation state of phosphorus increases after the reaction; the reverse is Reduction reaction. Addition reactions: another molecule adds to phosphorus; the reverse is dissociation/elimination reaction. Elimination reactions: one molecule leads to two molecules. Oxidative addition: the reaction combines both Oxidation and Addition reactions. Inorganic and Organic Reservoirs Organic phosphorus sources are the guano hills before the coast of Peru that developed by accumulation of bird excrements and that are determinedly excavated since the 19th century. Natural inorganic phosphorus deposits occur primarily as phosphate in the mineral apatite. Apatite is defined as a natural, variously colored calcium fluoride phosphate, Ca5F(PO4)3, with chlorine, hydroxyl, and carbonate sometimes replacing the fluoride Pathway of Movement The phosphorus cycle is the biogeochemical cycle that describes the movement of phosphorus through the geosphere, hydrosphere, and biosphere. Unlike the other major biogeochemical cycles (oxygen, carbon, nitrogen, and water), the atmosphere does not play a significant role in the movements of phosphorus because phosphorus and phosphorus-based compounds are usually solids at the typical ranges of temperature and pressure found on Earth. The phosphorus cycle reflects the harmonious interactions between organisms and their biotic and abiotic environments, with phosphorus flowing through each compartment by give and receive actions and allowing life to exist. However, sometimes the harmony that has been built up over many years is disrupted by human beings, who may put excessive amounts of phosphorus into a particular ecosystem through fertilizer, sewage, or other means. Movement Cont. Impacts of Human Intervention Human influences on the cycle mainly from use of commercial fertilizer The concentration of phosphorus in the fertilizer create a runoff effect when it rains, and is deposited in lakes, rivers, streams, etc, and leads to eutrophication. Eutrophication: Natural process by which lakes, streams, etc. receive excess nutrients that stimulate excessive plant growth. Impacts Cont. Humans can also alter the cycle in another way by cutting down the rain forest through the use of agricultural fertilizer. These runoffs provide most of the phosphate found in water, increase of phosphate in rivers and streams, along with other bodies of water. Creators Alyssa McCarrtt Aylsse Riggs Jenna McCarty Julie Davis







