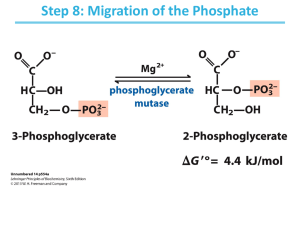
Biochemistry 304 2014 Student Edition Gluconeogenesis Lectures
advertisement

GLUCONEOGENESIS Student Edition 5/30/13 Version Dr. Brad Chazotte 213 Maddox Hall chazotte@campbell.edu Web Site: http://www.campbell.edu/faculty/chazotte Original material only ©2000-14 B. Chazotte Pharm. 304 Biochemistry Fall 2014 Goals •Learn the enzymes and step of the gluconeogenesis pathway. •Learn the similarities and difference the between gluconeogenesis and glycolysis pathways. •Understand the principles for biosynthetic (anabolic) pathways vs catabolic. •Understand how the gluconeogenesis pathway is regulated and how it is regulated vs glycolysis. •Understand the concept and benefits of metabolic burden sharing •Be aware the Cori cycle. •Understand how substrate cycles can amplify metabolic signals and/or produce heat. Biosynthetic (Anabolic) Pathways •Use chemical energy (ATP, NADH, NADPH) to synthesize cellular components from simple precursor molecules. •Generally reductive rather than oxidative. •Anabolism and catabolism proceed simultaneously in a dynamic steady-state to maintain the “intricate orderliness of living cells”. Lehninger 2000 p722 Organizing Principles of Biosynthesis 1 1. Molecules are synthesized and degraded by different pathways. a. Two opposing anabolic and catabolic pathways may share many reversible reactions. b. Each pathway has at least one unique and essentially irreversible reaction. c. This insures that the carbon flow through these pathways is dictated by the cell’s changing requirements for energy, precursors, and macromolecules rather than simple mass action. Lehninger 2000 p722 Organizing Principles of Biosynthesis 2 2. Corresponding anabolic and catabolic pathways are controlled by one or more reactions unique to each pathway. a. Opposing pathways are regulated in a coordinated, reciprocal manner so that the stimulation of the anabolic pathway is accompanied by the inhibition of the catabolic one and vice versa. b. Biosynthetic pathways are typically regulated at an early, exergonic step that commits intermediates to the pathway and does not waste energy by making unneeded intermediates. Lehninger 2000 p722 Organizing Principles of Biosynthesis 3 3. Energy requiring biosynthetic processes are coupled to the breakdown of ATP in such a way that the overall process is essentially irreversible in vivo. a. Thus the total amount of energy from ATP and NAD(P)H used always exceeds the minimum free energy needed to convert the precursor into the biosynthetic product. b. Consequently the biosynthetic process is thermodynamically favorable (G < 0) even for low precursor concentrations. Lehninger 2000 p722 Carbohydrate Biosyntheses from Simple Precursors The pathway from PEP to glucose-6-P is common to the biosynthetic conversion of many different precursors in animals and plants. Gluconeogenesis, meaning “formation of new sugar”, occurs in all animals, plants, fungi, and microorganisms. In most cases the reactions are essentially the same. Gluconeogenesis Lehninger 2000 Fig 20.1 Importance of Gluconeogenesis The brain depends on glucose as its primary fuel using ~120g/day out of ~160g/day for the typical adult human. Red blood cells use only glucose as a fuel. Only 20g are present in the body fluids and ~190 g are available from glycogen storage. Therefore, total reserves of glucose are about a single day’s supply. For longer periods of starvation, glucose must be formed from noncarbohydrate sources. Pyruvate Noncarbohydrate Precursors for Gluconeogenesis Any metabolite that can be converted to pyruvate or oxaloacetate can be a glucose precursor Lactate is formed by active skeletal muscle when glycolysis > oxidative metabolism. Lactate is converted by lactate dehydrogenase to pyruvate. Pyruvate (Certain) Amino acids are derived from protein in the diet or from muscle protein catabolism during starvation. Carbon skeletons of most amino acids are catabolized to pyruvate or citric acid cycle intermediates Glycerol is the result of a breakdown of triacylglycerols in fat cells. Fatty acids also result, but cannot be used by animals to make glucose. Glycerol enters glycolysis or gluconeogenesis at dihydroxyacetone phosphate Glycerol’s Entry in Gluconeogenesis or Glycolysis Gluconeogenesis Berg, Tymoczko & Stryer, 2012 Chap. 16 np.481 The Gluconeogenesis Balanced Equation 2Pyruvate + 2NADH +4H+ + 4ATP +2GTP +6H2O Glucose +2NAD+ + 4ADP + 2GDP + 6Pi Gº = –16 kJ/ mol Because of the presence of separate gluconeogenic enzymes at the three irreversible steps in the glycolytic pathway that converts glucose to pyruvate, glycolysis and gluconeogenesis both are rendered THERMODYNAMICALLY favorable. Gluconeogenesis Voet & Voet 1995 Chap 21 P.604 Gluconeogenesis Pathway Gluconeogenesis Berg, Tymoczko & Stryer, 2012 Fig. 16.24 Voet, Voet & Pratt 2013 Figure 16.15 Subcellular Location of Gluconeogenic Enzymes • Gluconeogenesis enzymes are cytosolic except: (1) Glucose 6-phosphatase (endoplasmic reticulum) (2) Pyruvate carboxylase (mitochondria) (3) PEPCK (cytosol and/or mitochondria) Mitochondrial pyruvate can: 1) be converted to citrate and used in the cytosol for the synthesis of fatty acids. 2) be changed to acetyl CoA and enter the citric acid cycle. 3) be converted by pyruvate carboxylase to oxaloacetate for gluconeogenesis. Horton et al., 2000 Chap 13.6 G’s of Erythrocyte Glycolytic Rxs Lehninger 2000 Table 20.1 Sequential Rx in Gluconeogenesis from Pyruvate Lehninger 2000 Table 20.2 Gluconeogenesis Pathway I Gluconeogenesis Berg, Tymoczko & Stryer, 2012 Fig. 16.24b Conversion of Pyruvate into Phosphoenolpyruvate Pyruvate carboxylase Pyruvate + CO2 + ATP + H2O oxaloacetate + ADP + Pi + 2H+ •The bypass of glycolysis’ pyruvate kinase reaction requires two separate reactions in gluconeogenesis. •One of the anaplerotic reactions that is used to maintain the levels of intermediates in the citric acid cycle Gluconeogenesis G’ = -2.1 kJ mol -1 Voet, Voet & Pratt 2013 Fig 16.16 Synthesis or PEP from Pyruvate I Gluconeogenesis Lehninger 2000 Fig 203a Pyruvate Carboxylase: Rx Mechanism Pyruvate Carboxylase catalyzes the ATPdriven synthesis of oxaloacetate from pyruvate and HCO3-. This reaction occurs in two phases. Phase I is a three step reaction sequence. Biotin is carboxylated at its N1’ position by a bicarbonate ion. Phase II in this phase the activated carboxyl group is transferred to pyruvate from carboxybiotin in a three step reaction sequence to form oxaloacetate. Gluconeogenesis Voet , Voet & Pratt 2008 Fig 16.18 Domain Structure of Pyruvate Carboxylase The ATP grasp domain activates HCO3- and transfers CO2 to the biotin-binding domain. From there the CO2 is transferred to pyruvate generated in the central domain. Gluconeogenesis Berg, Tymoczko & Stryer, 2012 Fig. 16.25 Pyruvate Carboxylase’s Biotin-binding Domain Key feature: biotin is on a flexible tether, attached to the -amino group of lysine, allowing it to move between the ATP-bicarbonate site and the pyruvate site. Gluconeogenesis Berg, Tymoczko & Stryer, 2012 Fig. 16.26 Carboxybiotin Berg, Tymoczko & Stryer, 2012 Fig. 16.27 Gluconeogenesis Voet, Voet & Pratt 2013 Fig 16.17b Opposing Pathways of Gluconeogenesis and Glycolysis II Gluconeogenesis Lehninger 2000 Fig 20.2b Conversion of Oxaloacetate into Phosphoenolpyruvate phosphoenolpyruvate carboxykinase Oxaloacetate + GTP Phosphoenolpyruvate + CO2 + GDP G’ = 2.9 kJ mol -1 Gluconeogenesis Synthesis or PEP from Pyruvate II Oxaloacetate is converted to phosphoenolpyruvate in the cytosol by PEP carboxykinase in a reaction that requires Mg2+ and GTP as the phosphoryl donor. Voet, Voet & Pratt 2013 Figure 16.19 Gluconeogenesis PEP Carboxykinase: Rx Mechanism A monomeric, 74 kD enzyme that catalyzes a GTP-driven decarboxylation of oxaloacetate to form PEP and GDP The CO2 that carboxylates pyruvate to synthesize oxaloacetate is eliminated in the formation of PEP. Gluconeogenesis Voet & Voet Biochemistry 1995 Fig. 21.5 The Mitochondrion Supplies Malate made from Pyruvate for use in Gluconeogenesis in the Cytosol Gluconeogenesis Note: the starting molecule is pyruvate. •Inside the mitochondrion oxaloacetate is reduced to malate in order to be transported outside the mitochondrion •Once the malate is transported outside (via the malate - -ketoglutarate shuttle) it is re-oxidized to oxaloacetate Berg, Tymoczko & Stryer, 2012 Fig. 16.28 Alternate Paths from Pyruvate to PEP This oxaloacetate is converted directly to PEP by a mitochondrial isozyme of PEP carboxykinase Pathway predominant when the starting molecule is pyruvate Gluconeogenesis When lactate is the precursor this pyruvate to PEP bypass is predominant Lehninger 2000 Fig 20.4 PEP & Oxaloacetate Transport Cytosol Mitochondria PEP has a direct transporter, whereas oxaloacetate does not and must be converted for transmembrane passage. Route 2 involves the conversion of oxaloacetate to malate AND involves NADH. Route 1 uses aspartate amino transferase. In this case oxaloacetate is converted to aspartate for transport. PEP is transported between the mitochondrion and the cell cytosol by a specific membrane transporter so it can move between the two compartments depending on the equilibrium. Voet, Voet & Pratt 2013 Figure 16.20 Sequential Reactions in Gluconeogenesis (Reprise) Gluconeogenesis Lehninger 2000 Table 20.2 Gluconeogenesis Pathway II Gluconeogenesis Berg, Tymoczko & Stryer, 2012 Fig. 16.24a Conversion of Fructose 1,6-bisphosphate into Fructose -6P and PPi: An Irreversible Step Fructose 1,6biphosphatase Fructose 1,6-bisphosphate + H2O Gluconeogenesis fructose 6-phosphate + Pi G’ = -16.3 kJ mol -1 Conversion of Glucose-6-phosphate into Glucose: An Irreversible Step Glucose-6-phosphatase Glucose-6-phosphate + H2O Gluconeogenesis Glucose + Pi G’ = -12.1 kJ mol -1 Glucose-6-Phosphatase This enzyme is found primarily in the endoplasmic reticulum of liver cells. – Why? Metabolic Control of Glycolysis and Gluconeogenesis In cells gluconeogenesis and glycolysis are coordinated such that one is mainly inactive while the other in highly active. (If both were highly active at the same time the result would be a FUTILE CYCLE consuming two ATP and two GTP per reaction cycle). •The rate of glycolysis is typically controlled by the glucose concentration. •The rate of gluconeogenesis is typically controlled by the concentrations of lactate and other glucose precursors. Reciprocal Regulation of Gluconeogenesis and Glycolysis in Liver IMPORTANT!! Gluconeogenesis Berg, Tymoczko & Stryer, 2012 Fig. 16.30 Voet, Voet & Pratt 2013 Fig. 16.21; 16.23 “Subtrate Cycle” can amplify metabolic signals and produce heat. Gluconeogenesis Berg, Tymoczko & Stryer, 2012 Fig. 16.34 Metabolic Burden Sharing Different organs/tissues in the body can be metabolically linked. Lactate produced by active skeletal muscle and erythrocytes is an energy source for other organs. •Skeletal muscle during vigorous exercise produces pyruvate at a rate faster than oxidative metabolism via the citric acid cycle can use it. •Also NADH production is more rapid than its conversion to NAD+ in aerobic metabolism. •(Remember that glycolysis needs NAD+ for glyceraldehyde 3-P oxidation to proceed.) •The cell can oxidize NADH to NAD+ in a reaction that converts pyruvate to lactate via lactate dehydrogenase. The problem is that lactate can not be further metabolized, only converted back to pyruvate. Important: Muscle’s ability to reduce pyruvate to lactate is a way shift the metabolic burden under high stress to other organs, e.g. liver, heart. (Reaction also regenerates NAD+ in muscle) Cori Cycle (an interorgan metabolic “pathway”) Lactate & pyruvate diffuse out of active muscle into blood. Excess lactate in the blood enters the liver where it is converted to pyruvate and then to glucose via gluconeogenesis. Gluconeogenesis: Cori Cycle Berg, Tymoczko & Stryer, 2012 Fig. 16.35 Horton et al., 2002 Fig. 13.12 Coordination of Glycolysis and Gluconeogenesis In liver and kidney glucose is produced by gluconeogenesis and can go out into the blood to be used by other tissues such as muscle. In muscle alanine is formed by a transamination reaction from pyruvate. Whereas the reverse process occurs in the liver. This cycling helps to maintain the nitrogen balance Muscles and red blood cells can produce lactate which, as we have seen in the Cori cycle can travel to the e.g. liver where it is metabolized to pyruvate, etc Gluconeogenesis (Remember: alanine is also a major glucose precursor) Berg, Tymoczko & Stryer, 2002 Fig. 16.34 Opposing Pathways of Gluconeogenesis and Glycolysis I Gluconeogenesis Lehninger 2000 Fig 20.2a End of Lectures