energy change
advertisement
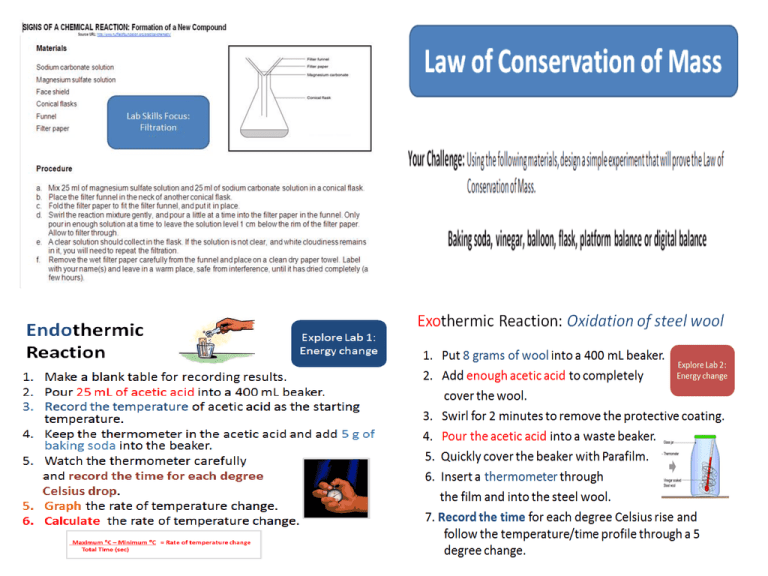
Lab Skills Focus: Filtration Chemical Equation MgSO4 + Na2CO3 → MgCO3(s) + Na2SO4 magnesium + sodium sulfate carbonate → magnesium + carbonate sodium sulfate Clear liquid Word Equation When solutions of two soluble salts are mixed, a solid may form. This solid is called a precipitate. and the reaction is called a precipitation reaction. Precipitation reactions are used to make insoluble salts. In this example the soluble salts are magnesium sulfate and sodium carbonate, and the insoluble salt formed is magnesium carbonate. Law of Conservation of Mass Instructions 1. Measure 5 g of baking soda and put into a flask. 2. Measure 20 mL of vinegar and carefully pour it into a balloon. 3. Carefully attach the balloon to the top of the flask without spilling the vinegar into the flask. 4. Place the flask with balloon on a weighing scale and record the mass of the system. 5. Tip the vinegar from the balloon into the flask and record your observations. 6. Measure the mass of the system after the reaction. 7. Pop the balloon and measure the mass again. Summary 1. Explain whether your results support the Law of Conservation of Mass. There are times when mass might appear to change during a chemical reaction. If you experience this during an experiment, be sure to remember the Law of Conservation of Mass. The change in mass must be accounted for in a way other than destroying or creating atoms. Endothermic and Exothermic Reactions (Heat Change) Exothermic and Endothermic Reactions An energy change accompanies the forming or breaking of a bond between atoms in a molecule. O Energy is released (produces heat) when a bond forms Exothermic o Energy is absorbed from the surroundings to break the bonds in a molecule Endothermic In your notebook: • • • • Draw a diagram of the set-up Make a data table Make a graph Calculate temperature change as a function of time Endothermic Reaction Explore Lab 1: Energy change 1. Make a blank table for recording results. 2. Pour 25 mL of acetic acid into a 400 mL beaker. 3. Record the temperature of acetic acid as the starting temperature. 4. Keep the thermometer in the acetic acid and add 5 g of baking soda into the beaker. 5. Watch the thermometer carefully and record the time for each degree Celsius drop. 5. Graph the rate of temperature change. 6. Calculate the rate of temperature change. Temperature/T degrees C Starting temperature- ex. Time/sec 28 27 26 25 24 23 Calculating Rate of temperature change Maximum ⁰C – Minimum ⁰C = Rate of temperature change Total Time (sec) Endothermic Reaction Explore Lab: Energy change Summary: Explain how this lab shows a chemical reaction. How does the graph show that the reaction is endothermic? Explain. Endothermic reaction results: • Starts at around 25 degrees C and ends at around 21 degrees C • Happens within about 2 minutes http://www.youtube.com/watch?v=MyAzjSdc3Fc Endothermic Reaction Exothermic Reaction: Oxidation of steel wool 1. Put 8 grams of wool into a 400 mL beaker. Explore Lab 2: Energy change 2. Add enough acetic acid to completely cover the wool. 3. Swirl for 2 minutes to remove the protective coating. 4. Pour the acetic acid into a waste beaker. 5. Quickly cover the beaker with Parafilm. 6. Insert a thermometer through the film and into the steel wool. 7. Record the time for each degree Celsius rise and follow the temperature/time profile through a 5 degree change. Exothermic reactions •The reaction produces heat. •In this reaction vinegar is used to remove the protective coating from steel wool, allowing it to rust. •When the iron combines with oxygen, heat is released. Exothermic Reaction: Oxidation of steel wool Summary: Explain how this lab shows a chemical reaction. On the same graph (endothermic), show a graph of temperature change as a function of time. How does the graph show that the reaction is exothermic? Explain. Exothermic Reaction: Oxidation of steel wool Applying the Law of Conservation of Mass: How many atoms of Oxygen is needed to react with 4 atoms of Iron to form 2 units of Iron oxide? 4 Fe + 4Fe + 2 Fe2O3 ___ O2 3O2 4 Fe + 6 O 2Fe2O3 = 4 Fe + 6 O