April 16 2013 Webinar - NanoRelease Food Additive
advertisement

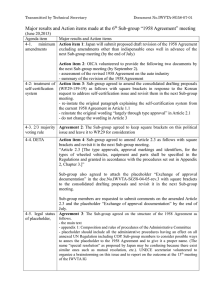
NanoRelease Task Group 1: Material Characteristics Characteristics Relevant to Uptake and Bioavailability www.riskscience.org 1 TG1: Material Characteristics CHARGE • Provide overview of physical and chemical attributes of nanoparticles that may affect their uptake in the alimentary tract. • Provide overview of physical and chemical attributes of the food matrix that may affect nanoparticle uptake in the alimentary tract. www.riskscience.org 2 Task Group 1: Sub-Groups Task Group 1 Material Characteristics Sub-Group 1 - What is (may be) in the food chain Sub-Group 2 – Nanomaterial properties Matrix interactions relevant to uptake & bioaccessibility www.riskscience.org 3 TG1 Members Sub-Group 1: ‘Catalogue’ Sub-Group 2: ‘Particle Properties’ CHAIR Scott Thurmond US FDA CHAIR Rickey Yada University of Guelph Tim Duncan US FDA CHAIR Neil Buck DSM Ltd Greg Noonan US FDA (CFSAN) Gemma Janer Leitat Technological Center Jeff Yourick US FDA (OARSA) Prabir Dutta Ohio State University Jamie Oxley Southwest Research Institute Cristina Sabliov Louisiana State University and LSU AgCenter Iseult Lynch University College Dublin Additional involved members/advisors: Anil Patri (US NIH), Jun Jie Yin (US FDA), Kevin Powers (U. of Florida), Lekh Juneja (Taiyo Kagaku Co, Japan), Sean Linder (US FDA), Il Je Yu (Hoseo University, S. Korea), Jonathan Powell (MRC Human Nutrition Research) Karen Tiede The (UK) Food & Environment Research Agency Julian McClements University of Massachusetts Chris DeMerlis ColorCon Inc. Andy Rao Cornell Food Science Michael Rogers Rutgers University Mengshi Lin University of Missouri Qingrong Huang Rutgers University Qixin Zhong University of Tennessee Yuan Yao Purdue University www.riskscience.org 4 Sub-Group 1: Catalogue Purpose of the Nanomaterial Catalogue • Review of ENM that are purportedly in internationally marketed food products • Provide “real time” input to NanoRelease task groups to support identification and development of analytical detection and characterization methods on nanomaterials used in commerce. Collection methods Resources: • FoodEssentials LabelBase, Gladson Nutrition database, Mintel Global New Products database • U. S. Patent and Trademark Office and European Patent Office databases • Project on Emerging Nanotechnologies consumer inventory • Published literature and business press • Threshold® professional literature/patent search firm • Regional sources www.riskscience.org 5 Sub-Group 1: Catalogue Results Nanomaterial Products Origin Sub-Group 1: Catalogue Summary • Catalogue is just a snapshot of what may be on the international market. • Two nanomaterials, calcium and silver, predominate, although nano-calcium was found only in Asia. • Supplements (nutritional and dietary) dominate the product classes for the incorporation of nanomaterials. Conclusions • Lack of labeling requirements in most countries make compilation of a comprehensive catalogue difficult. • Although this snapshot is focused primarily on North America and Asia, we feel that it may be representative of food-related nanoproducts found in other regions of the world. • Without analytical data for the identified nanoproducts, it is impossible to confirm that they contain nanomaterials. www.riskscience.org 7 Sub-Group 1: Catalogue NanoMaterials known to be present in the food chain www.riskscience.org 8 Sub-Group 2: Material Characteristics www.riskscience.org 9 Sub-Group 2: Material Characteristics Particles Metals and metalloids Polymeric encapsulates Emulsions, Dispersions and Powders Thereof www.riskscience.org 10 General properties of interest Chou, L. Y. T, K. Ming and W. C. W. Chan. Chem. Soc. Rev. 2011, 40, 233-245. www.riskscience.org 11 Digestive Processes & Food Interaction Sub-Group 2: Material Characteristics Nano Form Materials Physical Change Mucus Epithelium Vasculature & Local tissues lymphatics Transcellular Paracellular Persorption Disruption to molecular components Sequestration & elimination Cytosis & accumulation www.riskscience.org 12 Sub-Group 2: Material Characteristics Metals and Metalloids Definition and Usage: • Metal and metalloid particles in food are used for a wide range of applications: nutrients, colour additives, flow agents, food contact materials. Their nano-size may be intentional (e.g., to improve functionality as in the absorption of nutrients) or unintentional (portion of a particle population above the nano range). Requirements for Characterisation: • Concentration and chemical composition (core and surface) • Primary size (and surface are), shape, and aggregation/agglomeration/exfoliation state. • Surface charge Uncertainties: • Most relevant concentration units • At which point NM should be characterized. Prior to inclusion in food matrix, in the food matrix, in contact with gastrointestinal fluids… www.riskscience.org 13 Sub-Group 2: Material Characteristics Metals and Metalloids Analytical Gaps and Difficulties: • How to extract nanomaterials from food matrices without altering their properties, such as aggregation/agglomeration/exfoliation state. • Quantitative method for metal content exist (e.g., ICP-MS) but do not inform on the properties of the material (size, shape, surface coating). • Methods that allow the evaluation of size, shape, and aggregation state (e.g., TEM, RAMAN) are not quantitative, low throughput and expensive. Matrix Interactions: • Food matrices may affect the properties (and ultimately uptake) of nanomaterials, by changing their aggregation/agglomeration/exfoliation state, by changing their surface properties (by coating them), and when considering relatively soluble nanomaterials by determining their dissolution rate into ions. • Nanomaterials can also change the food matrix by modifying the bioavailability of some nutrients or chemically modifying them. www.riskscience.org 14 Sub-Group 2: Material Characteristics Polymeric Encapsulates Definition and Usage Polymeric nanoparticles with a typical size range of 20-1000 nm formed by a polymeric core, with the active component entrapped in the polymeric matrix, usually surrounded by a surfactant layer that stabilizes the system. Requirements for Characterisation Size, zeta potential, morphology, hydrophobicity, solubility, stability, degradation Uncertainties GI fate, nanoparticle degradation, uptake through the gut, biodistribution, metabolism, excretion, toxicity Analytical Gaps and Difficulties Nanoparticle-food matrix interaction, nanoparticle tracing in the gut and in the body Matrix Interactions Largely unknown www.riskscience.org 15 Sub-Group 2: Material Characteristics Emulsions, Dispersions & Powders Thereof Definition and Usage Preparations of water-immiscible nutrients and additives, designed for stabilisation, ease of handling, delivery or organoleptic properties. Various preparation methods for emulsions and dispersions in water are available, powder production involves the use of soluble biopolymer and spray-drying or other similar method. Requirements for Characterisation • • • Digestibility Particle size distribution Composition & Charge Uncertainties Whether there is direct absorption from the GI tract, thus circumventing normal physiological digestion. Analytical Gaps and Difficulties • • Sample preparation: what to model (as produced, as used, as prepared, GI environment) Representative number-size distribution, lack of analytical methods suitable for emulsions Matrix Interactions Exacerbation of the above due to interference from complex matricies ‘Black box’ ….. www.riskscience.org 16 Summary Requirement Key Points Difficulty Composition - - Surface physico-chemistry In what environment Multiple-steps Morphological aspects Where in the food-chain to characterise Multiple-steps Size (incl agglo/aggre) Appropriate unit Method to suit appropriate unit ‘Sample prep’ Preparation causes change Shape May not be homogeneous quantification Digestability Qualification & quantification of direct uptake? All of the above www.riskscience.org 17 Conclusion o As far as can be ascertained current ‘nano-sized’ ingredients are minerals, silver and new nutrient/additive preparations. o Silver, ceramics and clays are used in contact materials. o List of required measurands is limited. o However, almost all measurands present difficulty: what product stage should be considered? • matrix effects are mostly unknown. what method(s) is suitable for sample preparation and analysis? www.riskscience.org 18 Conclusion As manufactured As formulated As prepared As eaten As passaged in GI www.riskscience.org 19