Structure and distribution - 성균관대학교 이강노교수 천연물 약품화학
advertisement

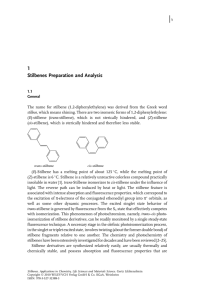
성균관대학교 약학대학 천연물 약품화학 실험실 석사 1기 김윤식 Stilbenoids are fonned by a particular branch of the flavonoid biosynthetic pathway. They are of special interest to natural product researchers for their roles in plant resistance to fungal pathogens and their biological effects. This review in the volume of Bioactive Natural Products provides a comprehensive account of the occurrence, chemistry, biological roles and activities of the stilbenoids. Nearly 800 stilbenoids, isolated from natural sources in the recent 12 years, are grouped into structural types and discussed in tenns of their reported phannacological activity. The major groups of stilbenoids which are discussed in detail include stilbenes, bibenzyls, bisbibenzyls, phenanthrenoids, stilbene oligomers etc. Detailed tables and figures list the occurrence of stilbenoids in major plant species, and methods used for extracting and analyzing stilbenoids are discussed. Biosynthetic pathways and chemical synthesis are reviewed and the biological activities of stilbenoids are also addressed. The coverage of the new structures is from 1994 to 2006. Stilbenoids were first isolated from the plants in 1899 and were named by Gorham in 1980. With the chemical diversity and expanded findings of their bioactivities, stilbenoids are attracting increasing interest across the world, especially after the hot findings of combretastatin A-4(816) and resveratrol(1), which shows promising chemo preventive effects against cancer. . A pro-drug of combretastatin A-4 (816), the water soluble phosphate derivative, is now in phase II of clinical trials. The number of stilbenoids found in plants, is now in excess of 1000, compared with just over 100 listed in 1980, and about 300 in 1995. . Herein, we review the recent progress in the studies of stilbenoids with respect to their structure, distribution, extraction and isolation, technologies used in structure identification, synthesis and biosynthesis, and the last but not the least, bioactivities Stilbenes Other stilbenoids Bibenzyls STILBENOIDS . Stilbene oligomers Bisbibenzyls Phenanthrenoids 2.1 Stilbenes Group A: simple stilbenes having oxygen functions on the aromatic rings, including the methylenedioxy derivatives and glycosides. Group B: prenylated and geranylated stilbenes, regardless of the substitution position and including the cyclized derivatives. . Group C: aryl benzofuran derivatives. Group D: carbon substituted stilbenes other than the prenylated and geranylated ones, the C-giycosides and those in group C. Group E: hybrid stilbenes can not be attributed to the above groups. 2.1 Stilbenes 2.1.1 Group A (Simple Stilbene) . (13) 3,4-Dihydroxy-3'-methoxystilbene (8-11) Phoyunbenes A-D Pholidota yunnanensis inhibitory effects on nitric oxide production liverwort Marchesini bongardiana 2.1 Stilbenes 2.1.1 Group A (Simple Stilbene) . 2.1 Stilbenes 2.1.1 Group A (Simple Stilbene) . 2.1 Stilbenes 2.1.1 Group A (Simple Stilbene) . 2.1 Stilbenes 2.1.1 Group A (Simple Stilbene) . 2.1 Stilbenes 2.1.1 Group A (Simple Stilbene) . 2.1 Stilbenes 2.1.2 Group B (Prenylated and Geranylated Stilbenes) . 3-(2,3-Dihydroxy-3-methylbutyl) resveratrol An enantiomeric mixture in a probable ratio of 5:3 for the 2"R and 2"S forms 2.1 Stilbenes 2.1.2 Group B (Prenylated and Geranylated Stilbenes) . 2.1 Stilbenes 2.1.2 Group B (Prenylated and Geranylated Stilbenes) 2.1 Stilbenes 2.1.3 Group C (aryl benzofuran derivatives) 2.1 Stilbenes 2.1.3 Group C (aryl benzofuran derivatives) 2.1 Stilbenes 2.1.4 Group D (Carbon Substituted Stilbenes Other Than Those in Groups 3 and 5) 2.1 Stilbenes 2.1.5 Group E (hybrids) Seeds of Aiphanes aculeata Willd Gnetum klossii 2.1 Stilbenes 2.1.5 Group E (hybrids) 2.1 Stilbenes 2.1.5 Group E (hybrids) Macaranga schweinfurthii (Euphorbiaceae) 2.2 Bibenzyls Liverworts are rich sources of bibenzyls. In this review, bibenzyls are classified into four groups: Group A : simple bibenzyls having oxygen functions on the aromatic rings, including the methylenedioxy derivatives and glycosides. Group B : prenylated, geranylated and farnesylated bibenzyls, regardless of the substitution position. Group C : 4-hydroxybenzyl substituted bibenzyls. Group D: other bibenzyls, including aryl-dihydrobenzofurans, phthalides, dihydroisocoumarins, etc. 2.2 Stilbenes 2.2.1 Group A (Simple Bibenzyls) Stemona collinsae roots 2.2 Stilbenes 2.2.1 Simple Bibenzyls (Group A) leaves of Empetrum nigrum Liverwort Plagiochila spinulosa 2.2 Stilbenes 2.2.1 Simple Bibenzyls (Group A) Scorzonera humilis (Asteraceae) 2.2 Stilbenes 2.2.2 Prenylated, Geranylated and Famesylated Bibenzyls (Group B) leaves of Glycyrrhiza lepidota Anti-viral activity 2.2 Stilbenes 2.2.2 Prenylated, Geranylated and Famesylated Bibenzyls (Group B) roots of Bauhinia malabarica Roxb 2.2 Stilbenes 2.2.3 4-hydroxybenzyl substituted bibenzyls (Group C) tubers of Pleione bulbocodioides 2.2 Stilbenes 2.2.4 Other Bibenzyls (Group D) 2.2 Stilbenes 2.2.4 Other Bibenzyls (Group D) 2.3 Bis(bibenzyls)s or bisbibenzyls Liverworts are rich sources of bisbibenzyls compounds. Liverworts are a group of plants belonging to the bryophytes. DNA analysis has revealed that liverworts are the earliest land plants. Since the isolation of marchantin A (812) from Marchantia and of riccardin A from Riccardia species by Asakawa et al., more than 100 macrocyclic . bisbibenzyls including their dimers have been isolated. So far, there have been nearly 70 bisbibenzyls identified since 1994. The occurrence of bisbibenzyls in both pteridophytes and liverwort is very important in determining the evolutionary ladder of both terrestrial spore-forming green plants. 2.3 Bis(bibenzyls)s or bisbibenzyls (239, 241) Taiwanese liverwort Reboulia hemisphaerica (242, 243) Marchantia chenopoda cultivated in Venezuela 2.3 Bis(bibenzyls)s or bisbibenzyls Chinese liverwort Marchantia polymorpha L. (Marchantiaceae) 2.3 Bis(bibenzyls)s or bisbibenzyls 2.3 Bis(bibenzyls)s or bisbibenzyls Pholidota yunnanensis 2.3 Bis(bibenzyls)s or bisbibenzyls 2.4 Phenanthrenoids 2.4.1 Phenanthrenes 2.4 Phenanthrenoids 2.4.1 Phenanthrenes wetland plant Juncus acutus 2.4 Phenanthrenoids 2.4.2 9,10-dihydroxyphenanthrenes underground parts of Stemona cf. pierrei 2.4 Phenanthrenoids 2.4.2 9,10-dihydroxyphenanthrenes The orchid Agrostophyllum callosum 2.4 Phenanthrenoids 2.4.2 9,10-dihydroxyphenanthrenes two species of Juncus genus 2.4 Phenanthrenoids 2.4.2 9,10-dihydroxyphenanthrenes 2.4 Phenanthrenoids 2.4.3 Dimeric Phenanthrenoids Allelocheinical interaction, between the wetland plant Juncus acutus and microalgae 2.4 Phenanthrenoids 2.4.3 Dimeric Phenanthrenoids Symmetrical dimers Unsymmetrical manner 2.4 Phenanthrenoids 2.4.4 Phenanthrene Alkaloids Naturally occurring aristolactams, aristolochic acids and dioxoaporphines; having a phenanthrene chromophore, are a small group of compounds mainly found in the Aristolochiaceae. They have also been reported from plants of the Annonaceae, Monimimaceae, Menispermaceae and Piperaceae. 2.4 Phenanthrenoids 2.4.5 Other Phenanthrenoids 2.5 Stilbene Oligomers Structural identification and NMR characterization of stilbene oligomers still remain a difficult task due to complicated structures and sometime confusing stereochemistry that comprises many diastereomers, epimers, and even example of rotational isomers. The distribution of stilbeneoligomers has been mainly found in the following families: Dipterocarpaceae, Gnetaceae, Vitaceae, Cyperaceae, Leguminosae, Moraceae, Welwitschiaceae, Umbelliferae, lridaceae, Celastraceae, Paeoniaceae and Haemodoraceae. They are usually accompanied by their monomers. Dipterocarpaceae, Gnetaceae and Vitaceae all constitute a significant number of oligostilbenes. 2.5 Stilbene Oligomers 2.5.1 Stilbene Dimers Gnetum montanum 2.5.1 Stilbene Dimers 2.5.1 Stilbene Dimers 2.5.1 Stilbene Dimers Maackia amurensls 2.5.2 Stilbene Trimers roots of Sophora leachiana 2.5.2 Stilbene Trimers Stem of Kobresia nepalensis (Cyperaceae) 2.5.2 Stilbene Trimers 2.5.2 Stilbene Trimers Vitis thunbergii Sophora davidii 2.5.2 Stilbene Trimers Foeniculum vulgare Miller 2.5.3 Stilbene Tetramers The genus Caragana of Leguminosae 2.5.3 Stilbene Tetramers X2 stems of Vilis davidii 2.5.3 Stilbene Tetramers Roots of Vitis amurensis 2.5.3 Stilbene Tetramers Stem bark of Shorea uliginosa 2.5.4 Other Oligomers Resveratrol pentamer Resveratrol hexamer Resveratrol octamer 2.6 Other Stilbenoids 2.6.1 Oligostilbene Derivatives 2.6 Other Stilbenoids 2.6.2 Flavonostilbenes Stem of Gnetum africanum and the root of G. gnemon 2.6 Other Stilbenoids 2.6.2 Flavonostilbenes Roots of Sophora a!opixuroides 2.6 Other Stilbenoids 2.6.3 Stilbenolignans 2.6 Other Stilbenoids 2.6.4 Others Root bark of Artocarpus rigida It is the development of separation and identification techniques that makes the isolation and elucidation of the stilbenoids applicable. Silica gel is still the most commonly used material of the stationary phase. However, many other materials possessing better resolution or different isolation mechanisms have also been widely applied, e.g. Sephadex LH-20, ODS C18, MCI gel CHP20P, Toyopearl HW-40F, especially the former two. The combination use of the materials is efficient for the separation of the structures with high similarity and high polarity such as stilbene glucoside sulfates. The application of high performance liquid chromatography (HPLC) is very common in the isolation and analysis procedures. The application of high performance liquid chromatography (HPLC) is very common in the isolation and analysis procedures. Other methods have also been successfully employed in the isolation procedure of stilbenoids, including MPLC, preparative TLC, centrifugal partition chromatography(CPC), and the support-free technique of multilayer coil countercurrent chromatography (MLCCC). A method based on capillary electrophoresis with electrochemical detection (CE-ED) was employed for the determination of oligomeric stilbenes found in the roots of Caragana species. The direct coupling of HPLC and 1H NMR spectroscopy is a straight forward, analytical technique which is being used more and more frequently to assign structures of natural products, avoiding time consuming isolation. Nowadays, LC-MS systems have been often applied to identify and quantify stilbenoids HPLC-CD experiments were carried out in the studies of isoplagiochins C and D (261, 262) and their analogs' stereochemistry NMR fingerprinting and GC-MS techniques have also been used in the ' analysis of bibenzyls. Eluent wall technique-overpressured layer chromatography (FEW-OPLC) has been successfully employed in the detection of red wine constituents High resolution NMR (especially 2D NMR techniques, e.g. HMBC, HMQC, HSQC, COSY, TOCSY, NOESY, ROESY etc.) and HR-MS are indispensable nowadays making the structure elucidation ' feasible and efficient. 4.1 X-Ray Crystallography X -ray crystallography is very useful to provide structural information and to solve the configuration confusions. Many structures of the stilbenoids have been established with the help of X-ray crystallography. 4.2 Conformational Studies The' coexistence of the enantiomeric conformations of macrocyclic bisbibenzyls was detected by X-ray crystallography, NMR and chiral HPLC experiments. Isoplagiochins C and D (261 and 262) are intriguing optically active molecules, which do not possess any kind of traditional chiral centers. 5.1 Biosynthesis of stilbenes 5.2 Biosynthesis of Stilbeneoligomers 5.3 Biosynthesis of Bisbibenzyls 5.4 Biosynthesis of 9,10-Dihydrophenanthrene Bibenzyls are bicyclic intermediates and require O-methylation as a prerequisite for their conversion into dihydrophenanthrenes 5.5 Biosynthesis of Other stilbenoids Lin et al. proposed that the seven artocarpols containing an oxepin ring stem from appropriate hydroxyl stilbenes Suzuki cross-coupling Heck coupling Perkins condensation 7.1 Anti-Microbial Activity 7.1.1 Antifungal liverwort Bazzania trilobata IC50 = (271)3.9, (267)4.0, (283)2.6 μg/mL 7. Anti-Microbial Activity 7.1.2 Antibacterial Artocarpus lakoocha (Monkey Jack) Antimycobacterial activity with the MIC values of 12.5 and 50 μg/mL 7. Anti-Microbial Activity 7.1.3 Anti-virus Leaves of Glycyrrhiza lepidota (American Licorice) Anti-viral activity with an EC50 of 2.0 μg/ml and an IC50 of 5.0 μg/ml 7. Anti-Microbial Activity 7.2 Anticancer Cytotoxicity assay is the most commonly used preliminary, anticancer protocol. The anticancer effects of the stilbenoids usually involve directing cytotoxicity, inhibition of the proliferation or inducing apoptosis of the tumor cells. 7. Anti-Microbial Activity 7.2 Anticancer In phase II of clinical trials 7. Anti-Microbial Activity 7.2 Anticancer 1000 times stronger than etoposide Potent inhibitory activity than daunorubicin eight times stronger than daunorubicin 7. Anti-Microbial Activity 7.3 Anti-lnflammation and Immunomodulating Activity 8-11 inhibited NO production in RAW 264.7 without cytotoxicity 7. Anti-Microbial Activity 7.3 Anti-lnflammation and Immunomodulating Activity Inhibitory effect on the formyl-peptide stimulated superoxide anion formation in neutrophils (IC50 26.0 μM) (IC50 20.9 μM) 7. Anti-Microbial Activity 7.4 Antioxidant Activity (1) reduce or diminish oxidative stress and provide cardiovascular protection Most stilbenoids possess antioxidant activities because they possess polyphenol functions in the molecules. Some of their beneficial effects, hepatoprotective action, cardiovascular protection, for instance, are in close relation to their antioxidant activities. 7. Anti-Microbial Activity 7.4 Antioxidant Activity 7. Anti-Microbial Activity 7.4 Antioxidant Activity Lipid peroxide inhibition and superoxide dismutases (SOD) like antioxidant activity 7. Anti-Microbial Activity 7.5 Phytotoxicity Many phenanthrenes and 9,10-dihydrophenanthrenes isolated by DellaGreca et al. from the Juncus genus, demonstrated allelochemical activity against algal pests. Gigantol and batatasin III as well as synthetic analogs were tested for phytotoxicity in axenic cultures of the small aquatic plant. Lemna pausicostata The results suggest that orchid bibenzyls may be good lead compounds for the development of novel herbicidal agents 7. Anti-Microbial Activity 7.6 Ecdysteroid Antagonistic Activity 7. Anti-Microbial Activity 7.7 Estrogenic/ Antiestrogenic Activities Resveratrol has a structural similarity to diethylstilbestrol, a synthetic estrogen. Therefore, the effect of resveratrol on estrogen receptors (ER) has been evaluated although the results obtained are inconsistent. 7. Anti-Microbial Activity 7.8 Neuroprotective (139) stilbostemin B 3'-β-D-glucopyranoside (140) stilbostemin H 3'-β-D-glucopyranoside (204) stilbostemin I 2“-β-D-glucopyranoside 7. Anti-Microbial Activity 7.9 Antiplatelet Activity Roots of Vitis thunbergii 7. Anti-Microbial Activity 7.10 Antidiabetic The antidiabetic-activity-guided fractionation of cultivated Korean Rhubarb rhizomes (Rheum undulatum) led to the isolation of desoxyrhapontigenin, which could inhibit postprandial hyperglycemia by 35.8%. Stilbenes desollyrhapontigenin and rhapontigenin from the rhizoma of Himalayan rhubarb displayed varying degrees of inhibition of yeast and mammalian intestinal a-glucosidase 7. Anti-Microbial Activity 7.11 Hepatoprotective and Hepatotoxic Activity Protective effect of hepatic injury powerful hepatotoxins 7. Anti-Microbial Activity 7.12 Spasmolytic Activity Bibenzyl and phenanthrenes have been found to possess smooth muscle relaxant effects 7.13 Other Antimutagenic activity 7. Anti-Microbial Activity 7.13 Other Stimulation of osteopath growth activity