INCOMPATIBILITIES IN PRESCRIPTION Definition of Incompatibility
advertisement
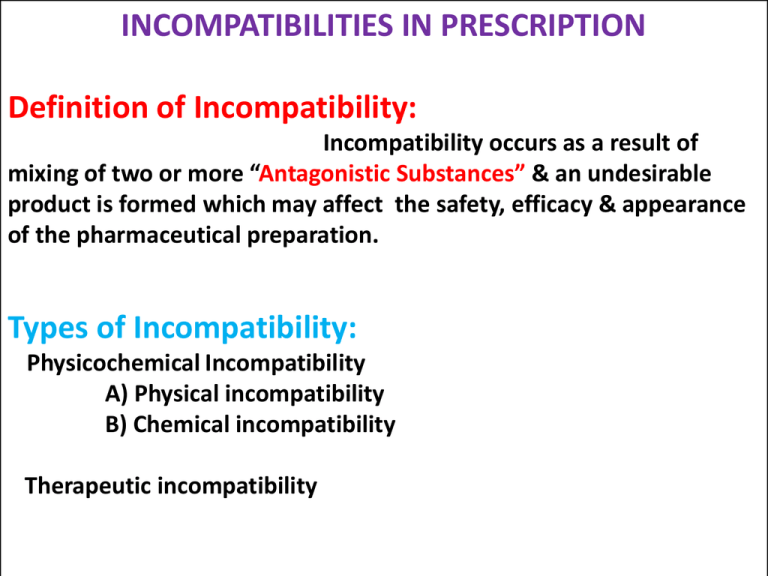
INCOMPATIBILITIES IN PRESCRIPTION Definition of Incompatibility: Incompatibility occurs as a result of mixing of two or more “Antagonistic Substances” & an undesirable product is formed which may affect the safety, efficacy & appearance of the pharmaceutical preparation. Types of Incompatibility: Physicochemical Incompatibility A) Physical incompatibility B) Chemical incompatibility Therapeutic incompatibility Physicochemical Incompatibilty If mixing two or more drugs or a drug & excipients, by particular method, results in a physicochemical change in the properties of drug or dosage form, or production of a new chemical substance having different pharmacological action, it is called as physicochemical incompatibility. If incompatibility is prevented by addition, substitution or elimination of one or more ingredient is called as adjusted incompatibility. General Methods used to remove incompatibilities. 1.Modify the order of mixing 2.Dispense with labels like Shake well before use 3.Recommend storage condition. 4.Add physical stabilizer. (Suspending or emulsifying agent) 5.Add chemical stabilizer. (Antioxidant/Buffer) 6.Add preservative. 7.Select soluble , compatible or stable form of dosage form. A] PHYSICAL INCOMPATIBILITY When two or more than two substances combined together a physical change takes place & unacceptable product is formed. Physical changes involves such as Immiscibility, Insolubility, Precipitation formation or liquefaction of solid materials. The physical incompatibilities may be corrected by using any one or more of the following methods: i) ii) iii) iv) v) Change the order of mixing of ingredients of the prescription. Emulsification Addition of suspending agent Change in the form of ingredients By addition, substitution or omission of therapeutically inactive sub.to help in compounding of the prescription. Example of Physical Incompatibility & Their Methods of Correction: 1.Immiscibility: Oils & Water are immiscible with each other. They can be made miscible with water by emulsification. Example: Rx Castor oil water upto 15ml 60ml Make an emulsion. To overcome this incompatibility an emulsifying agent is used to make a good emulsion. 2. Insolubility: It means the inability of material to dissolve in a particular solvent system. The liquid preparations containing Indiffusible solids such as chalk, aromatic chalk powder, acetyl salicylic acid, phenacetin, zinc oxide & calamine etc. Suspending agent - Increase the thickness of the preparation. -Uniform distribution of the insoluble substances which facilitating uniform measurement of each dose. E.g Acacia,Tragacanth,SLS,etc Example: Rx Phenacetin Caffeine Orange syrup Water upto 3g 1g 12ml 90ml Make a mixture. In this prescription phenacetin is an indiffusible sub. Compound powder of tragacanth or mucilage of tragacanth is used as a suspending agent to make a stable suspension. 3. Settling Suspending agent The drug in mixtures containing insoluble solids(Suspension), during storage settles at the bottom. Insoluble solids are of two types. Diffusible Solids – Kaolin & light magnesium carbonate remains evenly distributed. Indiffusible solids – Chalk , Calamine --- Has to increase viscosity of vehicles by adding suspending agent like gum tragacanth. 4. Poor Wettability Levigating agent Some drugs are poorly wetted with given vehicle hence these may float or sink at the bottom of vehicle. Hence levigation of these solids with wetting agent such as glycerine, propylene glycol, or hydrophilic surfactant helps in the uniform distribution of hydrophobic drug in water. Eg. Sulphur lotion. 5. Precipitation: A drug in solution may be precipitated , if the solvent in which it is insoluble is added to the solution e.g. resins are insoluble in water. Volatile oils are soluble in alcohol. When water is added into the alcoholic solution of volatile oil, the non aromatic portion of the oil get precipitated & turbidity appears. Cap – locking Co-solvents Liquid preparations especially those containing syrups may show crystallization of sugar in the closure , resulting in cap locking. It can be minimized by adding co-solvents like glycerine, propylene glycol or sorbitol 6. Grainy Semisolids Non uniform cooling Partial solidification of higher melting point waxes may occur when cool spatula is used for mixing or when a hot product is poured in a cool container. Grains are also developed during preparation of creams, if the aqueous phase & oil phase do not have same temperatures at the time of mixing. This can be prevented by avoiding localized cooling. 7. Liquefaction: Eutectic mixture If low melting point solids are mixed together, a liquid or soft mass known as ‘Eutectic mixture’ is produced. This occurs due to the lowering of the melting point of mixture to below the room temp. & liberation of water of hydration. e.g camphor, menthol, thymol. Liquefication can be corrected by i) Dispensing individual ingredient separately ii) Compounding powder using diluents such as lactose, magnesium oxide or magnesium hydroxide are separately mixed with eutectic substances and such mixtures are mixed to produce final product. This prevents physical contact of liquifiable substances. iii) Allowing completion of liquefaction & absorbing the liquid using adsorbent such as substances like kaolin. B] CHEMICAL INCOMPATIBILITY DEFINITION: It may be as a result of chemical interactions between the ingredients of a prescription & a toxic or inactive product may be formed. It is due to oxidation-reduction, acid base hydrolysis or combination reactions. These reactions may be noticed by effervescence, decomposition, colour change . Chemical incompatibilities are of following types. 1. Precipitation: •Precipitation of the drug takes place due to - i. pH change ii. A chemical reaction between drug-drug or drug-additives. •When the ppt product is therapeutically active it is formulated as per the procedure used to prepare mixtures containing diffusible & indiffusible solid. •If the resultant ppt is inactive or toxic the formulation should be rejected. a)pH Change: • Most of the medicines are often salts of weak acids & weak bases. • The unionized forms are insoluble in water & ionisable salts are soluble in water. • A pH change, not only changes solubility, but it also changes rate of degradation. • If the resultant precipitate has therapeutic value & it is chemical stable, one can tolerate the incompatility. • A suitable co-solvent can be used to increase solubility, otherwise a suspension is formulated. • Eg. Morphine hydrochloride above 2.5 % concentration , is insoluble & results in the formation of diffusible precipitate under alkaline condition. • The solubility of precipitated morphine in alcohol is 1 in 100. • Thus incompatibility can be treated either by – i) Preparing suspension of diffusible precipitate Ii) Using alcohol as co-solvent to prepare solution. Eg. 2 ) Caffeine citrate above 2.2 % concentration, under alkaline condition produces indiffusible precipitate but when alcohol is used, it tolerates a higher concentration of caffeine citrate without precipitation. b) Precipitation by chemical reaction: i) Drug-drug interactions: Eg. Caffeine Citrate • Active ingredients react with other drugs or additives yielding diffusible or indiffusible precipitate. • Caffeine citrate is a mixture of equal weight of caffeine & citric acid. • The citric acid reacts with sodium salicylate to liberate salicylic acid in the form of precipitate. • It causes gastric irritation. • Hence only caffeine is used, it forms a soluble complexes with sodium salicylate. • Therefore, caffeine citrate should be replaced with half the amount of caffeine to get clear solution. Flavour• Liquorice looses its flavour due to precipitation in presence of acid. • Acid reacts with glycyrrhizin, a flavouring constituent of liquorice forming glycyrrhizic acid precipitate. 2.Redox reaction: Some drugs or their dosage forms undergo oxidation when exposed to air, excessive temperature & due to over dilution of liquids, incorrect pH or presence of catalyst. The common catalyst includes metal ions, enzymes & bacteria. The drug in the liquid state is more sensitive to oxidation than insoluble state . a) Auto oxidation of oils, fats, phenolic substances, aldehydes & vitamins. •This can be prevented by addition of primary antioxidants such as alpha tocopherol, or BHT. b) Paraaldehyde, tannins, epinephrine, sulphacetamide & related compounds undergo oxidation activated by heat. •Hence for such drugs requires against trace metal ions. •This can be achieved by adding antioxidants such as ascorbic acid, sodium metabisulfite or complexing agents like EDTA are used to control oxidation. c) Preparations containing riboflavin, folic acid & ascorbic acid show incompatibility. Riboflavin is light sensitive, and easily degraded by heat and light. Hence should be stored at its stable pH 6 to 6.5. D) When dry powders contains both oxidising & reducing agents, the mixture may explode. •The inter-particulate friction developed during mixing increases chances of redox reaction. •Therefore such reacting substances should be dispensed separately or powders should be mixed lightly by spatulation method. 3. Hydrolysis •Hydrolysis can be controlled by avoiding moisture contact or by changing pH. •Aspirin is more sensitive to water & gets converted to more irritant acetyl salicylic acid. Hence it is granulared without use of water. •Paracetamol is stable between ph 5 & 7 . •While ibuprofen shows more solubility above pH 6. Therefore compound suspension of these two drugs in combination pH of solution is 5-6. 4. Racemisation The conversion of an optically active form to an optically inactive form without changing chemical constitution usually results in reduced therapeutic activity. 5. Effervescence : Two or more ingredients of formulation reacts to generate CO2, To overcome such reactions either a) Mix the reacting ingredients in open container & allow to complete reaction before filling in to container. The rate of reaction can be increased by using hot water. b) Change the one or more reacting ingredients. c) Dispense reacting substances seperately 6. Colour change The colour change is visible incompatibility. When the colour reaction is rapid it is allowed to complete before dispensing. The delayed color change in formulation creates confusion in the mind of patient. Eg. Sodium Salicylate mixture BPC During storage the alkaline solution undergoes oxidation by atmospheric oxygen & becomes brownish black. The coloured product is safe and has therapeutic value but it may create confusion in the mind of patient Hence anti-oxidant like sodium metabisulphite is added as antioxidant to control oxidation. 7. Incompatibilty with containers: The product filled in a container may react chemically with the container or get absorbed by the container or the closure. i) Glass containers alkali leaching. ii) Rubber closures may adsorb preservatives. iii) Metal containers catalyse rate of chemical reaction. THERAPEUTIC INCOMPATIBILITY It is result of prescribing certain drugs to a patient with the intention to produce a specific degree of p’cological action, but nature or intensity of the action produced is different from that intended by the prescriber. Therapeutic incompatibility is due to : A) Medication errors B) Drug interactions A) Medication Errors: Medication errors done by doctors, pharmacists, nurses & patients. Medication errors can be categorized as – 1. Prescription errors 2. Dispensing errors 3. Selection errors 4. Bagging errors 5. Administration errors 1. Errors in prescription writing The prescription should be neatly & correctly written by the medical practitioner, otherwise it is major hassle for the pharmacists. -Spelling mistakes. - Illegible handwriting. a) SALA Medicines(Sound Alike Look Alike): Tab. Dulcolax a laxative tablet & Tab . Duoclox an antibacterial tablet. b) Type of dosage form: Same drug can be available in tablet, capsule or liquid oral dosage forms for adults, drops for pediatric patients. Selection of dosage form is based on ability of patients to take that medicines. C) Strength of Medicines : Some medicines, especially those whose dosing is critical , are available in different strengths. Eg. Haloperidol , antipsychotic drug available in the form of tablets of various strength viz. 0.25, 2mg, 5mg,10mg, 20 mg. d) Quantity to be dispensed: Pharmacists should check the dose & see that the quantity of medicine is sufficient to complete a course. e) Dose & Direction: Pharmacists should check the dose, dosage regimen & direction to use. 2. Dispensing Errors: a)Poor handwriting b)Long prescription c)Incomplete patient information d)Deviation in attention of pharmacist e)Misunderstanding of verbal orders. 3. Selection of medicine •While removing medicines from shelves pharmacist should be alert. •He should remove the correct medicine. •SALA medicines should not be kept adjacent to one another. 4. Bagging Errors: After billing, dosage forms should be packed in appropriate bags or covers & handed over to the right person. 5. Errors in administration i) Breaking the coated or sustained release tablets. ii)Suspension administration without shaking. iii)Taking medicament at wrong time before or after meal. B) Drug interactions 1. Contra – indicated drugs: There are certain drugs which may be contraindicated in a particular disease or a particular patient who is allergic to it. Example: the penicillin & sulpha drugs are contraindicated to the patients who are allergic to it. 2.Synergistic & antagonistic drugs: Many drugs exhibit Synergism & Antagonism when administered in combination. When the two drugs are prescribed together, they tend to increase the activity of each other, called as synergism. Example: A combination of aspirin & paracetamol increase the analgesic activity. When two drugs having the opposing p’cological effects are called antagonism. Example: Acetyl salicylic acid and probenecid c)Unintentional drug interaction The effect of one drug is altered by the prior or simultaneous administration of another drug. Tetracycline hydrochloride 250mg Direction –take one capsule with milk Tetracycline inactive by calcium which is present in milk. d) Intentional drug interaction Rx Acetophenetidin 150mg Acetyl salicylic acid 200mg Caffeine 30mg Send 10 capsules. Acetophenetidin & acetyl salicylic acid are analgesic. Acetophenetidin depresses the CNS & this effect is undesirable. Caffeine is CNS stimulant to neutralise the side effect of acetophenetidin.