Organic Chemistry ppt 2012
advertisement

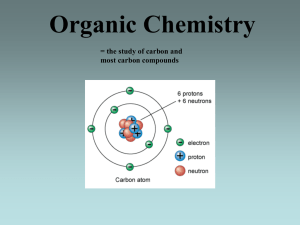
Organic Chemistry = the study of carbon and most carbon compounds Bonding of Carbon Atoms Carbon atoms have a tendency to covalently bond with other carbon atoms and form chains. Straight chains Branched chains Ring chains Carbon atoms are able to form up to four covalent bonds: **Remember: Carbon has 4 valence electrons. Carbon atoms can engage in single, double, or triple covalent bonds: saturated compounds = contain only single bonds unsaturated compounds = contain at least 1 double or triple bond Saturated and Unsaturated fatty acids This double bond between the two carbon atoms makes this organic compound unsaturated. Molecular vs. Structural Formulas Molecular Formulas – show the atoms and the number of atoms involved in a molecule but nothing else (ex: propane is C3H8 ) Structural Formulas – show each type of atom and how they are arranged in a molecule. The Condensed Structural formula of propane is CH3CH2CH3 Molecular Formula Structural Formula Condensed Structural Formula CH4 CH4 C 2 H6 CH3CH3 Hydrocarbons = organic compounds that contain only atoms of hydrogen and carbon Homologous series of hydrocarbons: (a) Alkanes = contain only single covalant bonds - General formula: CnH2n+2 (b) Alkenes = contain one double covalent bond - General formula: CnH2n (c) Alkynes = contain one triple covalent bond - General formula: CnH2n-2 Naming Organic Compounds (also see Mr. Caiafa’s ppt) Naming straight-chained hydrocarbons: Use Reference Table P (Organic Prefixes) and Table Q (Homologous Series of Hydrocarbons) to name & write the formulas. When naming alkenes & alkynes, indicate where the double/triple bond is located in the molecule. The double bond is located on the 1st carbon…so its name would be: 1-butene **The carbons are numbered so as to keep the number for the double bond as low as possible** Both compounds have four carbons (use prefix but-) and a double bond (use ending –ene) The double bond is located on the 2nd carbon…so its name would be: 2-butene Both compounds have four carbons (use prefix but-) and a triple bond (use ending –yne) The triple bond is located on the 2nd carbon…so its name would be: 2-butyne The triple bond is located on the 1st carbon…so its name would be: 1-butyne Naming Organic Compounds Naming branched hydrocarbons: 1) Find the longest carbon chain which contains the functional group or multiple bond if present and name it (using Tables P & Q to find correct prefix & ending). 2) Number the longest chain (left to right or right to left) so that the functional group/multiple bond/longest side chain (branch) is on the lowest numbered carbon possible. 3) Name each side group but change the ending to -yl. 4) Use a prefix di-, tri-, tetra-, etc. to denote how many side groups of each length are present. 5) Before naming the side group give the number of the carbon to which the side group is attached. 6) Arrange the side groups in alphabetical order ignoring the prefixes di-,tri-, etc. Examples: 3.) The side group has only one carbon, so use the prefix methand add the ending –yl: methyl. 1.) The longest chain has 5 carbons, so the prefix pent- must be used. 2.) There are only single bonds, so the ending –ane must be used. Name: 3-methyl pentane 4.) Since the side group is right in the middle, the carbons can be numbered from either side. The methyl group is located on the 3rd carbon. 3.) Each side group has only one carbon, so use the prefix methand add the ending –yl: methyl. Since there are 3 methyl groups, use the prefix tri-: trimethyl. 1.) The longest chain has 4 carbons, so the prefix butmust be used. 2.) There are only single bonds, so the ending –ane must be used. Name: 2,2,3-trimethyl butane 4.) Count carbons so that the longest side chain has the lowest #. The first 2 methyl groups are located on carbon 2, and the next methyl group is located on carbon 3. Isomers = compounds with the same molecular formula, but different structural arrangements **As the # of carbon atoms in a compounds increases, the # of possible isomers also increases.** Example of Isomers: All of these compounds have the molecular formula C5H12 except for compound (4) which is C5H10 Functional Groups = atoms or groups of atoms that can replace hydrogen atoms in a hydrocarbon and give the compound distinctive physical and chemical properties (1) Halides: = when any of the halogens (F, Cl, Br, or I) replaces a hydrogen atom in an alkane - named by citing the location of the halogen attached to the chain and adding the appropriate prefix (fluoro-, chloro-, bromo-, or iodo-) Note: Table R provides examples on how to recognize and name compounds w/ each of the functional groups! (2) Alcohols: = one or more hydrogen atoms of a hydrocarbon are replaced by an –OH group (called a hydroxyl group) Note: The –OH group does not dissociate, and therefore alcohols - named by citing the location of the –OH are not bases/electrolytes. However, the –OH group does make group and changing the ending to –ol. alcohols polar molecules. - Classifying alcohols: Monohydroxy alcohol: one –OH group Dihydroxy alcohol: Trihydroxy alcohol: two –OH groups three –OH groups - Alcohols can also be classified according to the position of their –OH group: PRIMARY (1o): the functional group is bonded to a carbon that is on the end of the chain. SECONDARY (2o): The functional group is bonded to a carbon in the middle of the chain. TERTIARY (3o): The functional group is bonded to a carbon that is itself directly bonded to three other carbons. (3) Aldehydes: = the carbonyl group (-C=O) is found on the end carbon and is bonded to a H atom - named by substituting –al in place of the final –e of the corresponding alkane name (4) Ketones: = the carbonyl group (-C=O) is found on an interior carbon atom that is attached to two other carbon atoms - named by replacing the final –e from the corresponding alkane with –one; if necessary, cite which carbon atom the carbonyl group is attached to. (5) Ethers: = two carbon chains are joined together by an oxygen atom with single bonds to two carbon atoms - named by first naming the two methyl groups, followed by the word ether (when both R groups are the same, use prefix di-) (6) Organic Acids (carboxylic acids) are weak acids: = contain the carboxyl functional group (-COOH) - named by replacing the –e in the corresponding alkane name with –oic acid (7) Esters: = have the type formula R-CO-OR’ (R-CO-O- part of formula comes from an organic acid; the R’ part comes from an alcoholsee Esterification) - named for the alcohol and organic acid that make up the ester (8) Amines: = formed when one or more of the hydrogen atoms of ammonia are replaced by an alkyl group (ex CH3NH2 is methanamine) - named by changing the alkane ending of –e to –amine and then numbering the alkane chain to show the location of the amine group (9) Amides: = a compound formed by the combination of an amine with a carboxylic acid; recall from biology that amide groups are formed when amino acids condense to form a peptide bonds (See Condensation reaction) - named by changing the carboxylic acid acid reactant ending –oic acid with -amide Organic Reactions (also see Mr. Caiafa’s ppt) **Note: Generally occur more slowly than inorganic reactions. When covalently bonded substances react, they must first break relatively strong existing bonds before making new bonds.** (1) Combustion: = Hydrocarbons burn in the presence of oxygen to produce water and carbon dioxide (2) Substitution: = involves the replacement of one or more of the hydrogen atoms a saturated hydrocarbon with another atom or group (3) Addition: = involve adding one or more atoms at a double or triple bond Examples: Ethene (CH2=CH2) and Chlorine (Cl2) react to form 1,2-dichlorethane 2-Butene (CH3CH=CHCH3) and Hydrogen (H2) react to form Butane (CH3CH2CH2CH3) in (4) Esterification: = the reaction between an organic acid and an alcohol to produce an ester plus water Organic Acid + Alcohol Ester + Water (5) Saponification: = when an ester reacts with an inorganic base to produce an alcohol and a soap (6) Fermentation: = a chemical process in which yeast cells secrete the enzyme zymase and break down sugar into carbon dioxide and two carbon fragments of alcohol (7) Polymerization: = the formation of large polymer molecules Polymers = organic compounds make up of chains of smaller units covalently bonded to each other (a) Addition polymerization = involves the joining of monomers of unsaturated compounds (b) Condensation polymerization = involves the joining of monomers by removing water from hydroxyl groups and joining the monomers by an ether or ester linkage Addition Polymerization: Condensation Polymerization: H2O First 10 Alkanes in Series Hydrocarbon Molecular Formula Methane CH4 Ethane C 2H 6 Propane C 3H 8 Butane C4H10 Pentane C5H12 Hexane C6H14 Heptane C7H16 Octane C8H18 Nonane C9H20 Decane C10H22 First 10 Alkenes in Series Hydrocarbon Molecular Formula Ethene C 2H 4 Propene C 3H 6 Butene C 4H 8 Pentene Hexene Heptene Notice: There is no alkene corresponding to the methane of the alkane series. That is b/c there must be at least 2 carbon atoms to form a double bond. C5H10 C6H12 C7H14 Octene C8H16 Nonene C9H18 Decene C10H20 First 10 Alkynes in Series Hydrocarbon Molecular Formula Ethyne C 2H 2 Propyne C 3H 4 Butyne C 4H 6 Pentyne Hexyne Heptyne Notice: There is no alkyne corresponding to the methane of the alkane series. That is b/c there must be at least 2 carbon atoms to form a triple bond. C5H8 C6H10 C7H12 Octyne C8H14 Nonyne C9H16 Decyne C10H18