Organic Chemistry PowerPoint
advertisement

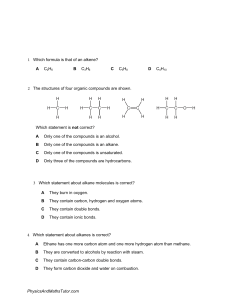
Organic “Carbon” Chemistry Chapter 13-14 Science 10 CT03D01 Resource: Brown, Ford, Ryan, IB Chem Organic “Carbon” Chemistry Chemistry for you, Lawrie Ryan Chapter 13 Pages 159-177 Hydrocarbons, Fossil Fuels, Distillation of Crude Oil, Cracking, Plastics, Polymers Chapter 14 Pages 178-185 Alcohols, Isomers, Ethanol, Alcohol Reactions, Carboxylic Acids 13.1 - Hydrocarbons A hydrocarbon is a compound containing only hydrogen and carbon Crude Oil, which is very important to the survival Venezuela is a mixture of many hydrocarbons Not only vital for fuels but also the starting materials for plastics and other polymers 13.1 - Alkanes The most common hydrocarbon found in crude oil is an alkane An alkane contains a ‘backbone’ of single-bonded carbon atoms and is saturated with hydrogen atoms Natural gas, methane, CH4, is the shortest alkane Alkane Methane, CH4 Ethane, C2H6 Propane, C3H8 Butane, C4H10 Pentane, C5H12 Hexane, C6H14 Heptane, ______ Octane, _______ 13.2 – Fossil Fuels Most common fuels are fossil fuels Coal, crude oil, natural gas, etc Coal, although it’s not a hydrocarbon, does contain carbon and hydrogen, as well as oxygen in some of it’s molecules From organic material (like trees) that died and were buried below swamps Crude oil, hydrocarbons Formed from tiny animals and plants which lived in the sea Takes millions of years to form fossil fuels In reality the energy comes from the sun to produce fossil fuels, but it simply takes so long to produce 13.2 – Finding Oil Crude oil today was made from mainly plankton that died about 150 million years ago. Their bodies did not decay normally due to lack of oxygen and with high pressures and temperatures, formed oil and natural gas. We can find oil by surveying the land and it’s topography Look for dome shaped layers (cap rock or anti-cline) Seismic survey 13.3 – Distilling Crude Oil When crude oil reaches the refinery it’s a thick, black, and smelly liquid This liquid contains long hydrocarbon chains At the refinery the long chains can be sorted out into groups of useful substances called fractions We can separate these substances by fractional distillation which separates substances based on their boiling point Fraction Length Color Thickness Reactivity Low BP (up to 80C) Short Clear Runny Easily lit (flammable, clean flame) Medium BP (80- Medium 150C) Yellow Thicker Harder to light, some smoke High BP (above 150C) Dark orange Thick Long Difficult to light, smoky flame 13.4 – Fractional Distillation in Industry Fraction Length of Carbon Chain Petroleum gas C1-C4 Petrol C4-C12 Kerosine C11-C15 Diesel C15-C19 Lubricating Oil C20-C30 Fuel Oil C30-C40 Bitumen C50 + 13.5 - Cracking After distillation of crude oil companies are still left with long hydrocarbons and the need is for shorter chains like petrol The solution is cracking meaning big molecules are broken down by heating them over a catalyst This is competed inside a cracker 13.6 - Plastics When oil companies crack large molecules into smaller ones, ethene is made Ethene is just like ethane, but with a double bond making it unsaturated vs ethene This ethene molecule is the starting material for plastics. When the double bond is broken, new bonds can form between several molecules forming polymers Lots of small, reactive molecules called monomers join together to make a polymer 13.7 – Ethene and the Alkenes Alkenes, which are also hydrocarbons, are very similar to alkanes, but are not saturated. They have at least one double bond and less hydrogen atoms which makes them unsaturated. Their names end in –ene instead of –ane Contain double bonds Alkane Methane, CH4 Very reactive Ethane, C2H6 Building block for polymers Propane, C3H8 Also react with Br, Cl, I, F water Strong acids Water and sulfuric adid Alkene Ethene, C2H4 Propene, C3H6 Butane, C4H10 Butene, C4H8 Pentane, C5H12 Pentene, C5H10 Hexane, C6H14 Hexene, C6H12 Heptane, ______ Heptene, ______ Octane, _______ Octene, _______ 13.8 - Polymerization There are two types of reactions that make polymers Addition – where at least two things simply join together Condensation – where water is given off in the process of joining molecules. Also known as dehydration synthesis 13.8 – Addition Polymerization Addition Reactions Monomers have at least one double bond The polymer is the only material formed in the reaction Easiest example is ethene used to make poly(ethene) n C2H4 -[-C2H4-]-n Where n = large number The double bonds open up to form single bonds to the adjacent monomer R can be just about anything 13.9 – Condensation Polymerization Nylon is an example of a polymer formed through condensation Fumes are given off as the different monomers react together. These small molecules given off could be H2O, HCl, etc. It depends on the ends of the monomers. The monomers have reactive parts at both ends and join end-to-end to make long chain polymers + H2O 13.10 – Properties of Plastics Many materials are made out of plastics PVC piping, bags, surfaces, protective films, bottles, etc Plastics often have advantages over the use of metal compounds and cost much less When we run out of oil we will also run out of access to cheap plastics This is why recycling our plastics is so important!! Chapter 14 – Organic Molecules Nomenclature! How do we name organic compounds? Alkane vs alkene Saturated vs unsaturated Functional groups Length of chain 14.1 – Types of Organics Types of Organic Molecules Saturated •Compounds which contain only single bonds •For example: alkanes Aliphatics •Compounds which do not contain a benzene ring; may be saturated or unsaturated •For example: alkanes, alkenes Unsaturated •Compounds which contain double or triple bonds •For example: alkenes, arenes Arenes •Compounds which contain a benzene ring; they are all unsaturated compounds •For example: benzene, phenol 14.2 - Members of Homologous Series Differ by a CH2 Can be represented by the same general formula Show gradation in physical properties Have similar chemical properties 14.2 - Members of Homologous Series… … differ by a –CH2 group 14.2 - Members of Homologous Series… … can be represented by the same general formula Formula Name CH4OH Methan-1-ol C2H5OH Ethan-1-ol C3H7OH Propan-1-ol C4H9OH Butan-1-ol C5H11OH Pentan-1-ol C6H13OH Hexan-1-ol C7H15OH Heptan-1-ol C8H17OH Octan-1-ol 14.2 - Members of Homologous Series… … show gradation in physical properties Alkane Boiling Point Methane, CH4 -164 Ethane, C2H6 -89 Propane, C3H8 -42 Butane, C4H10 -0.5 Pentane, C5H12 36 Hexane, C6H14 69 Heptane, C7H16 98 Octane, C8H18 125 Since the series differ by one –CH2 they have successively longer carbon chains Results in gradual trend of phy. Props Not always a linear growth Density and viscosity are other examples 14.2 - Members of Homologous Series… … show similar chemical properties As the have the same functional group Ex.1 – the alcohols have a functional –OH group, which can be oxidized to form organic acids Ex. 2 – the –COOH functional group, present in the homologous series of the carboxylic acids, is responsible for the acidic properties of these compounds 14.3 – Organic Formulas Emperical formula Simplest whole number ratio Ethane CH3 Molecular formula Actual number of atoms Ethane C2H6 Structural Formula Full, condensed, steriochemical 14.3 - Emperical Formula The simplest whole number ratio of the atoms it contains. For example, the emperical formula of ethane, C2H6, is CH3. This formula can be derived from percentage composition data obtained from combustion analysis. It is, however, of rather limited use on it’s own, as it does not tell us the actual number of atoms in the molecule. 14.3 - Molecular Formula Actual number of atoms of each element present. For example, the molecular formula of ethane is C2H6. It is therefore a multiple of the emperical formula, and so can be deduced if we know both the emperical formula and the relative molecular mass Mr. 14.3 - Full Structural Formula Graphic formula or displayed formula – shows every bonded atom. Usually 90o and 180o angles are used to show the bonds because this is the clearest 2-D representation, although it is not the true geometry of the molecule 14.3 - Condensed Structural Formula Often omits bonds where they can be assumed, and groups atoms together. It contains the minimum information needed to describe the molecule nonambiguously – in other words there is only one possible structure that could be described by its formula. 14.4 – IUPAC Nomenclature Nomenclature for Organic Compounds: the IUPAC system International Union of Pure and Applied Chemistry Rule 1: Identify the longest straight chain of carbons Rule 2: Identify the functional group Rule 3: Identify the side chains or substituent groups 14.4 - IUPAC Rule 1: Longest Chain # C atoms in longest Stem in IUPAC name Example 1 meth- CH4 methane 2 eth- C2H6 ethane 3 prop- C3H8 propane 4 but- C4H10 octane 5 pent- C5H12 pentane 6 hex- C6H14 hexane Note: ‘straight chain’ does not mean just 180o angles or unbranched chains of carbon atoms. Be careful, do not be confused by the way the molecule may appear on paper because of free rotation around the carbon-carbon single bonds. Example, all three below are the same…. 15.4 - IUPAC Rule 2: Functional Group The functional group is described by a specific ending (or suffix) to the name, that replaces the –ane ending of the name of the parent alkane. The suffixes used for some common functional groups are in the slides to follow. Those marked * will have slides with further information. 14.4 - Functional Groups Homologous Series Suffix in IUPAC name Example of compound Alkane -ane C3H8 propane Alkene -ene CH3CH=CH2 propene Alcohol -anol C3H7OH propanol chloro, Fluoro, bromo chloromethane Halogen Functional Group -Cl -F -Br 14.4 - IUPAC Rule 3: Side Chains Side Chain Prefix in IUPAC Example of Compound -CH3 methly- CH3CH(CH3)CH3 2-methylpropane -C2H5 ethly- CH(C2H5)3 3-ethlypentane -C3H7 proply- CH(C3H7)3 4-propylheptane -F , -Cl , -Br , -I fluoro- , chloro- , bromo- , iodo- CCl4 Tetrachloromethane -NH2 amino- CH2(NH2)COOH 2-aminoethanoic acid 14.5 - Structural Isomers Different arrangements of the same atoms make different molecules Molecular formula shows the atoms that are present in a molecule, but gives no information on how they are arranged. Consider, for example, C4H10 Each isomer is a distinct compound, having unique physical and chemical properties. 14.5 - Structural Isomers of Alkenes 14.6 - Alkanes as Fuels Release significant amounts of energy when they burn, highly exothermic, because large amount of energy released when forming.. Double bonds of CO2 Bonds in H2O C3H8 + 5O2 3CO2 + 4H2O ΔH = -2200 kJ/mol However, when O2 is limited….. 2C3H8 + 7O2 6CO + 8H2O when O2 is extremely limited….. C3H8 + 2O2 3C + 4H2O These are examples of the incomplete combustion of fossil fuels which makes them an environmental concern