View Poster - Idaho EPSCoR
advertisement
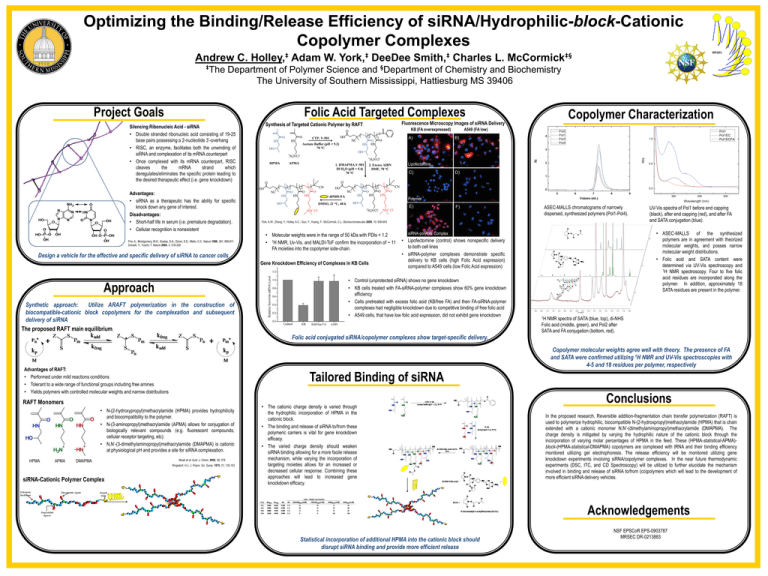
Optimizing the Binding/Release Efficiency of siRNA/Hydrophilic-block-Cationic Copolymer Complexes Andrew C. Holley,‡ Adam W. York,‡ DeeDee Smith,‡ Charles L. McCormick‡§ ‡The Department of Polymer Science and §Department of Chemistry and Biochemistry The University of Southern Mississippi, Hattiesburg MS 39406 Folic Acid Targeted Complexes Silencing Ribonucleic Acid - siRNA • Double stranded ribonucleic acid consisting of 19-25 base pairs possessing a 2-nucleotide 3’-overhang • RISC, an enzyme, facilitates both the unwinding of siRNA and complexation of its mRNA counterpart • Once complexed with its mRNA counterpart, RISC cleaves the mRNA strand which deregulates/eliminates the specific protein leading to the desired therapeutic effect (i.e. gene knockdown) Synthesis of Targeted Cationic Polymer by RAFT Copolymer Characterization Fluorescence Microscopy Images of siRNA Delivery KB (FA overexpressed) A549 (FA low) Pol1 Pol1EC Pol1ECFA 1.0 Abs Project Goals Lipofectamine 0.5 0.0 Advantages: • siRNA as a therapeutic has the ability for specific knock down any gene of interest. Disadvantages: • Short-half life in serum (i.e. premature degradation). • Cellular recognition is nonexistent Fire, A.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Nature 1998, 391, 806-811 Dorsett, Y.; Tuschl, T. Nature 2004, 3, 318-329 Polymer York, A.W.; Zhang, Y.; Holley, A.C.; Guo, Y.; Huang, F.; McCormick, C.L. Biomacromolecules 2009, 10, 936-943 • Molecular weights were in the range of 50 kDa with PDIs < 1.2 • 1H NMR, Uv-Vis, and MALDI-ToF confirm the incorporation of ~ 11 FA moieties into the copolymer side-chain. Gene Knockdown Efficiency of Complexes in KB Cells Synthetic approach: Utilize ARAFT polymerization in the construction of biocompatible-cationic block copolymers for the complexation and subsequent delivery of siRNA The proposed RAFT main equilibrium. 500 UV-Vis spectra of Pol1 before end capping (black), after end capping (red), and after FA and SATA conjugation (blue). • ASEC-MALLS of the synthesized polymers are in agreement with theorized molecular weights, and posses narrow molecular weight distributions. • Folic acid and SATA content were determined via UV-Vis spectroscopy and 1H NMR spectroscopy. Four to five folic acid residues are incorporated along the polymer. In addition, approximately 18 SATA residues are present in the polymer. siRNA-polymer Complex • Lipofectomine (control) shows nonspecific delivery to both cell lines • siRNA-polymer complexes demonstrate specific delivery to KB cells (high Folic Acid expression) compared to A549 cells (low Folic Acid expression) • Control (unprotected siRNA) shows no gene knockdown • KB cells treated with FA-siRNA-polymer complexes show 60% gene knockdown efficiency • Cells pretreated with excess folic acid (KB/free FA) and then FA-siRNA-polymer complexes had negligible knockdown due to competitive binding of free folic acid • A549 cells, that have low folic acid expression, did not exhibit gene knockdown 400 Wavelength (nm) ASEC-MALLS chromatograms of narrowly dispersed, synthesized polymers (Pol1-Pol4). Design a vehicle for the effective and specific delivery of siRNA to cancer cells Approach 300 1H NMR spectra of SATA (blue, top), di-NHS Folic acid (middle, green), and Pol2 after SATA and FA conjugation (bottom, red). Folic acid conjugated siRNA/copolymer complexes show target-specific delivery. Copolymer molecular weights agree well with theory. The presence of FA and SATA were confirmed utilizing 1H NMR and UV-Vis spectroscopies with 4-5 and 18 residues per polymer, respectively Advantages of RAFT: • Performed under mild reactions conditions • Tolerant to a wide range of functional groups including free amines • Yields polymers with controlled molecular weights and narrow distributions Tailored Binding of siRNA RAFT Monomers • N-(2-hydroxypropyl)methacrylamide (HPMA) provides hydrophilicity and biocompatibility to the polymer. • N-(3-aminopropyl)methacrylamide (APMA) allows for conjugation of biologically relevant compounds (e.g. fluorescent compounds, cellular receptor targeting, etc). • N,N’-(3-dimethylaminopropyl)methacrylamide (DMAPMA) is cationic at physiological pH and provides a site for siRNA complexation. HPMA APMA DMAPMA Moad et al. Aust .J. Chem. 2005, 58, 379 Ringsdorf, H.J. J. Polym. Sci. Symp. 1975, 51, 135-153 siRNA-Cationic Polymer Complex • The cationic charge density is varied through the hydrophilic incorporation of HPMA in the cationic block. • The binding and release of siRNA to/from these polymeric carriers is vital for gene knockdown efficacy. • The varied charge density should weaken siRNA binding allowing for a more facile release mechanism, while varying the incorporation of targeting moieties allows for an increased or decreased cellular response. Combining these approaches will lead to increased gene knockdown efficacy. Conclusions In the proposed research, Reversible addition-fragmentation chain transfer polymerization (RAFT) is used to polymerize hydrophilic, biocompatible N-(2-hydroxpropyl)methacrylamide (HPMA) that is chain extended with a cationic monomer N,N’-(dimethylaminopropyl)methacrylamide (DMAPMA). The charge density is mitigated by varying the hydrophilic nature of the cationic block through the incorporation of varying molar percentages of HPMA in the feed. These (HPMA-statistical-APMA)block-(HPMA-statistical-DMAPMA) copolymers are complexed with tRNA and their binding efficiency monitored utilizing gel electrophoresis. The release efficiency will be monitored utilizing gene knockdown experiments involving siRNA/copolymer complexes. In the near future thermodynamic experiments (DSC, ITC, and CD Spectroscopy) will be utilized to further elucidate the mechanism involved in binding and release of siRNA to/from (co)polymers which will lead to the development of more efficient siRNA delivery vehicles. Acknowledgements Statistical incorporation of additional HPMA into the cationic block should disrupt siRNA binding and provide more efficient release NSF EPSCoR EPS-0903787 MRSEC DR-0213883











