Unit 3 - Photosynthesis
advertisement

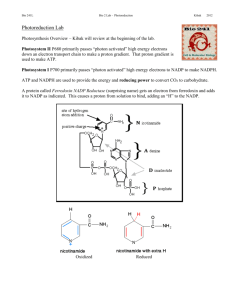
Module 3 - Photosynthesis Week 1 Introduction: Photosynthesis • Energy inherent in photons of light perform useful work: – Cleaves covalent bond in water to form O2 – High-energy electrons transferred to NADPH and ATP • Drives synthesis of glucose through anabolic pathway Three stages of photosynthesis • All occur in organelles called chloroplasts • Two stages depend on light absorption by chlorophyll – Photosystem I (P700) – Photosystem II (P680) • Third stage is Calvin cycle, and is independent of light Redox reactions in thylakoid membrane • PS-II e- transfer: chlorophyll PQ cyt b6f PC – PS-II also generates protons for electrochemical gradient • PS-I e- transfer: chlorophyll multi-subunit complex reduction of NADP+ to NADPH • Proton gradient generates ATP via ATP synthase Measuring photosynthetic rate • Multiple ways to assess the rate at which light energy converted into useful chemical energy – Electron transfer (what we will study) – Formation of ATP – Change in pH in lumen Hill reaction General equation Use of colorimetric reagent as electron acceptor DCPIP DCPIP • Electron transfer during photosynthesis can be interrupted by adding an artificial electron acceptor (e.g., DCPIP) – Acceptor must have lower energy than donor – Colorimetric compounds change colors upon accepting/donating an electron Quantifying photo-reduction activity of chloroplasts with colorimetric DCPIP assay • Spinach chloroplasts prepared for you • DCPIP used to assay rate of photo-reduction as determined in spectrophotometer • Use of a “blank” to calibrate spec • Positive and negative controls included • Test effects of a herbicide on rate of photoreduction Use of spectrophotometer • Monochromator tunes the wavelength of light passing through sample • Amount of light transmitted through sample measured by photodetector – 100% transmission = no absorption of light by sample – 0% transmission = sample absorbs all light • DCPIP is initially blue color reagent and absorbs light at 600 nm • As DCPIP accepts electrons it is converted to a colorless compound (no absorption of light) • Increase in light transmission indicates electron transfer from photosystem components to DCPIP Set up Spectronics 20+ • Set up for Spectronics 20+ • Turn on spec (knob on front face on left). Allow to warm up for 15-20 minutes. • Set wavelength to 600 nm (knob on top) • Adjust filter to 600-950 nm (bottom left) • Set spec to 0% T with nothing in the compartment – Close lid of empty compartment (no cuvette, no light) – Adjust left knob to 0% T • Set spec to 100% T with blank – Fill cuvette (glass 0.5” diameter tube) with water. Make sure to use at least 4 mL (about half-way filled). – Insert cuvette in compartment, close lid. – Using the front right knob, adjust to 100%T • Remove blank cuvette. • Insert cuvette containing sample and read %T Set up your different samples Table 1 Tube 1 Tube 2 Tube 3 No C'plasts 3 1 1 0 Dark C'plasts 3 1 1 0 Light C'plasts 3 1 1 0 Tube 4 Light C'plasts + Herbicide 2.5 1 1 0.5 water Rxn buffer 65 µM DCPIP Herbicide Chloroplasts? 0 0.3 0.3 Numbers are volume of each solution to add. Units=millimeters (mL) 0.3 • Number the glass cuvettes 1 through 4 and place them in a tube rack. • Fill each of the tubes with the indicated amount of water: – P1000 pipette (dial set to 1.00) with a blue tip attached • • • • Add 1 ml Reaction Buffer to each of the four tubes; P1000 set to 1000 μl Add 1 ml of DCPIP to each tube. To Tube #4, add 0.50 ml of your assigned herbicide. Add a small piece of Parafilm to seal the top of each tube, and invert several times to mix. • Visually inspect the height of the liquid in each of the four tubes; they should all be the same if you pipette your ingredients correctly; they should also all have the same bluish color. • Wrap the tube to be kept in the dark (Tube #2) in foil to block out incident light. Start your experiment • At Time = 0 (check the clock time or start a timer), add 300 µL of the chloroplast preparation to your Tube. Immediately cover with Parafilm and invert/mix the contents. • As soon as possible after adding chloroplasts to your tube and mixing, measure the % Transmission of the Tube in the spec. Record the Time and the %T in Table 6.3. • For Tubes #1, #3, and #4, place the Tubes under the light provided on your lab bench. Keep Tube #2 in darkness (wrapped in foil). • Each person in your group should do one Tube (stagger 5 min): – Note exact time chloroplasts were added, – Note time at which each measurement was made – Record Time and %T. • Repeat your measurement of the %T at various times from 1 to 16 minutes. • Be sure the dark Tube #2 is returned into foil after each measurement. Data collection Table 2 % Transmission measurements Tube 1 Time (min) 1 2 4 8 16 Tube 2 Tube 3 Tube 4 Light No C'plasts Dark C'plasts Light C'plasts C'plasts + Herbicide Data analysis with Excel Write up answers to questions 1. 2. 3. 4. 5. Compare the time course you obtained for samples with no chloroplasts with that of chloroplasts in the light. What happened to the absorbance at 600 nm in the tubes with chloroplasts? What specifically is occurring at the level of individual molecules to produce the effects you have observed? Compare the time course for samples with chloroplasts kept in light versus those kept in darkness. Explain what is happening at the molecular level and how it produced the observed results. Each lab group tested a different herbicide to determine if it inhibited photosynthetic redox reactions. Consult with the other groups in the lab: Which compound(s) appear to inhibit electron transport? Which do not? If more than one altered electron transport, rank them in relative order of inhibition. Some of the herbicides you tested might not have affected electron transport. What are some other potential targets for herbicides? (Hint: Most commercially available herbicides are not particularly toxic to animals because they interfere with processes that are unique to plants.) What other environmental factors or conditions do you think could increase or decrease the rate of redox reactions by chloroplasts? What is your rationale? Create your own experiment… • Discuss potential experiments • Consult with faculty/TA about your proposed experiment • Fill out “My proposed experiment” form and turn it in before leaving