electrochem
advertisement

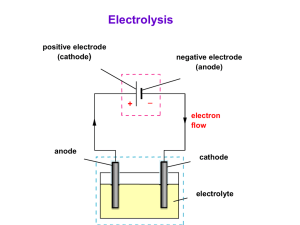
electrochem more fun facts and concepts Spontaneity and Electrochemistry • For those wondering how to determine spontaneity in redox reactions, this is how to do it: • We know that ΔG° determines spontaneity and we can manipulate the equation used to determine it to incorporate E°. • The equation we can use is ΔG°=-nFE°, where n is the mols of electrons exchanged, F is Faraday’s constant (96500 C/mol of e-), and E° is the standard potential. • In case E is given instead of E°, we can find ΔG instead with ΔG=-nFE. Spontaneity and Electrochemistry • Suppose we want to find E° for a cell, but one of the two reactants is an unknown compound. However, let’s say that these two reactants are at their standard state. • If we know their equilibrium concentrations at the standard state we can find E°. Spontaneity and Electrochemistry • To do this, we must manipulate the equation ΔG°= -nFE°. • Think back to entropy. ΔG° also equals –RTlnK. • So if ΔG°= -RTlnK and ΔG°= -nFE°, than we can say that -RTlnK = -nFE°. • From that we can derive that E° = RTlnK/-nF • As you can see, K is the only real unknown here and solving for it is easy enough given the concentrations at equilibrium. Spontaneity and Electrochemistry • A useful picture to have when talking about ΔG° and E° is: More Manipluations! • Suppose we have reactants that are not at their standard states. We can’t use standard reduction potentials to find E° of the cell. Instead, we must find E of the cell. • In this case, we’ll have to think back to ΔG and relate it to ΔG°. The equation for that is ΔG = ΔG° + RT ln Q. • We know that ΔG°= -nFE° and ΔG=-nFE, therefore we can change ΔG = ΔG° + RT ln Q to nFE = -nFE° + RT ln Q. More Manipulations! • If we divide both sides of that equation by -nF, we can get the equation E = E° - RTlnQ/nF. • Since we are using E°, the temperature will be 25°C or 298K. • This is a very famous equation called the Nernst Equation, which was made by a guy named Walther Nernst. Electrolysis • What is electrolysis? • Electrolysis is the process in which electrical energy is used to cause a nonspontaneous chemical reaction to occur. • An example of electrolysis is running a current through water in order to produce hydrogen gas. Electrolysis • Generally you will have to know electrolysis when a question asks how many mols of grams of something is produced when subjected to a current. • The current most commonly used is Amperes, which are coulombs per second. • Along with the information that 1 mole e- = 96,500 C, we can easily apply the idea of electrolysis. Electrolysis • Let say we have a solution of NaCl and we, for some reason, want Na(s). Suppose we run a current of 15 Amperes through the solution for 2 minutes. How many grams of Na(s) will we get? • First of all, 2 minutes is really 120 seconds and 15 Amperes is really 15 Coulombs/sec. Thus, in 120 seconds, we ran a total of 120 * 15 coulombs or 1800 coulombs through the solution. Electrolysis • Given that 1 mole e- = 96,500 C, we can now easily find out how many mols of e- we ran through the solution. • 1800C/96500C/mols e- = .018653 mols e- . • Since you only need one mol of electrons to reduce Na+, there are .018653 mols Na(s). • To find grams of Na(s), just divide the mols by the molar mass, or .018653/22.99 = 8.11E-4g Na(s) So that’s that. We blame Apple for our aesthetically pleasing, yet still really boring looking PowerPoint. We also blame G because he made us teach this to you kids.