Moles
advertisement
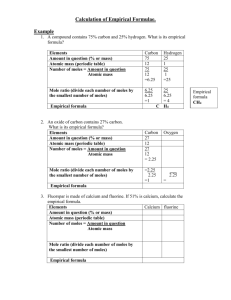
Masses of Atoms (Part 1) Relative Atomic Mass (Ar) tells us the mass of an atom compared to Carbon-12. The Ar takes into account all the isotopes of the element, so that is why Chlorine is Cl35.5. We can use relative atomic masses to work out the relative formula mass (Mr) of a molecule or compound. If we add up all the RAM nummbers present in a molecule or compound we get the Relative Formula Mass Example: 2 X Hydrogen + 1 X Oxygen H2O = = 2X1 + 1 X 16 = A formula mass of 18 Ar Numbers (NH4)3PO4 Solution: Split into 2 halves 1) (NH4)3 N = 14 X 1 H =1X4 NH4 = 18 (NH4)3 = 18 X 3 = 54 2) PO4 P = 31 X 1 O = 16 X 4 PO4 = 95 54 + 95 = a formula mass of 149 Calculate the relative formula mass of the following compounds (showing your working!): (a) Carbon monoxide CO (c) Sulphur dioxide SO2 (d) Calcium carbonate CaCO3 (e) Sodium hydroxide NaOH (f) Sulphuric acid H2SO4 (g) Copper sulphate CuSO4 Extension Activity (h) Lead nitrate Pb(NO3)2 (i) Calcium hydroxide Ca(OH)2 Part 2: The Mole Units of Measurement: We use units of measurement in life to give us an easier way to see large numbers. metre = 100 cm, litre = 1000 ml, kilogram = 1000 g The mole is a unit of measurement In chemistry we use it to tell us how many atoms or molecules there are The Mole Example: If I had 1 mole of grapefruits I would have 602,214,150,000,000,000,000,000 (6.02 X 1023) grapefruits which would be the same size as If I had 1 mole of H2O I would have 6.02 X 1023 molecules of water. How big is a mole? • A mole of sand grains would cover the United States in approximately one centimetre of sand. • A human body contains roughly one hundred trillion cells; there are roughly six billion people on Earth; so the total number of human cells on the planet is very close to one mole. • If you had exactly one mole of sheets of paper, you could make one million equal stacks from sea level on the earth that would pass the sun. • If you had a mole of pennies, you could give out enough money to everyone in the world so that they could spend a million dollars every hour, day and night, for the rest of their lives. The Mole If I could weigh 6.02 X 1023 molecules of water it would weigh 18g Q) What is the formula mass of water? 18 Q) What is the relationship between the formula mass and 1 mole of a chemical? 1 mole of a chemical is equal to the Formula Mass in grams Example I have 40g of NaOH The Formula Mass (FM) = 40 Therefore I have 1 mole NaOH 1 mole 2 mole 0.5 moles 0.25 moles etc = 40g = 80g = 20g = 10g Task • What is the relative formula mass of Magnesium chloride? • What is the relative atomic mass of Na? • What is the mass of 0.2 moles of sulphur? • What is the mass of 0.25 moles of CaCO3 • How many moles in 14 g of silver? • How many moles in 0.56 g of CaO? Triangle to help you: Atomic mass or Formula mass Part 3 Percentages of elements in compounds • Write down the Ar of every element in the cmpound. • Divide each one by the total Ar. • Times each one by a hundred. • E.g. Magnesium oxide – Mg = – Oxygen = Part 4: Empirical Formula • The empirical formula is the simplest ratio of the different atoms in it. For example for ethane it is CH3. • You can work out the empirical formula of a compound if you know the mass of each element in it. Example: What is the empirical formula of a compound that contains 27.3% carbon and 72.7% oxygen by mass? • Step 1 Find, and write down the relative atomic masses of the elements involved. • Ar (C) = 12 and Ar (O) = 16 • Step 2 Turn the percentages into grams. • There is 27.3g of carbon and 72.7g of oxygen • Step 3 Work out how many moles of each element this must be using number of moles =mass Ar • number of moles of carbon= 27.3 =2.28 12 • number of moles of oxygen = 72.7=4.55 16 • Step 4 Divide both numbers of moles by the smallest number (2.275 in this case). • number of moles of carbon = 2.28 = 1 2.28 • = number of moles of oxygen = 4.55 =2 2.28 In the empirical formula, there are 1 carbon atom and 2 oxygen atoms, so this is …….. Example, A compound contains 75g carbon and 25g hydrogen. What is its empirical formula? C H Amount 75 25 Convert to moles ( /Ar) /12 = 6.25 /1 = 25 Divide by the smallest number 6.25/ 6.25 25/6.25 =1 =4 C H4 Empirical formula 1 (a) Attempt: An oxide of carbon contains 27% carbon and 73% oxygen. What is its empirical formula? (this was the first example, so don’t do again if you have taken good notes!) 1. (b) Confirm: Fluorspar is made of calcium and fluorine. If 51% is calcium, calculate the empirical formula. 2. (a) Attempt: 1.68g of iron is combined with 0.48g of oxygen. What is the empirical formula of the new compound ? 2. (b) Confirm: 2.70g of aluminium is combined with 10.65g of chlorine. What is the empirical formula of the new substance? 3. Challenge: Saltpetre is a potassium salt. It is 13.9% nitrogen ,38.6% potassium and 47.5% oxygen. What is its empirical formula?