Hydroxyapatite (HA)
advertisement
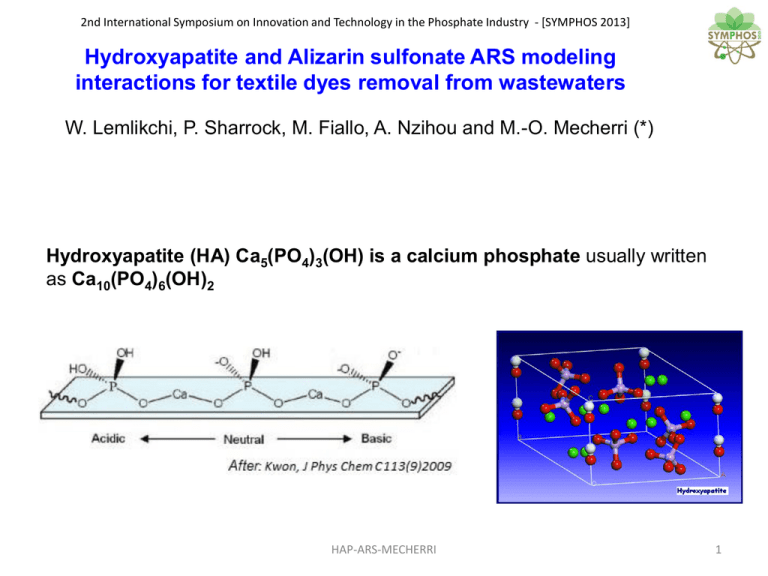
2nd International Symposium on Innovation and Technology in the Phosphate Industry - [SYMPHOS 2013] Hydroxyapatite and Alizarin sulfonate ARS modeling interactions for textile dyes removal from wastewaters W. Lemlikchi, P. Sharrock, M. Fiallo, A. Nzihou and M.-O. Mecherri (*) Hydroxyapatite (HA) Ca5(PO4)3(OH) is a calcium phosphate usually written as Ca10(PO4)6(OH)2 HAP-ARS-MECHERRI 1 2nd International Symposium on Innovation and Technology in the Phosphate Industry - [SYMPHOS 2013] HA has multiple exchange capacities inside its structure, including formation of vacancies to accommodate ions with other valencies, in particular carbonate ions and alkali and other metal ions. Hydroxyapatite (HA) is more than an adsorbent and was previously used for eliminating heavy metals in water pollution by adsorption and ion exchange. HA was also used for eliminating textile dyes from waste waters. Our previous works showed that HA can be regenerated after calcination, and reused several times. HAP-ARS-MECHERRI 2 2nd International Symposium on Innovation and Technology in the Phosphate Industry - [SYMPHOS 2013] Alirarin red sulfonate (ARS) sodium 3-alizarin-sulfonate (also called Alizarine Carmine, Alizarine Red S, Alizarine S) is a typical dihydroxyanthraquinone that has been used as a traditional natural dye originally derived from the roots of plants of the madder genus, also used a histochemical stain for calcium in bone tissues . HAP-ARS-MECHERRI 3 2nd International Symposium on Innovation and Technology in the Phosphate Industry - [SYMPHOS 2013] Textile mordant dyes such as alizarin generally contain a functional ligand capable of reacting strongly with Al(III), Cr(III), Co(II), Cu(II), Ni(II), or Fe(II), Fe(III) salts to give different colored complexes with the textile. Alizarin could therefore be used as a model compound for more complex dyes. The adsorption of alizarin red has long been used to assess the performance of the apatite before use in a water treatment facility. This led us to investigate the way calcium ions interact simultaneously with ARS and phosphate. HAP-ARS-MECHERRI 4 2nd International Symposium on Innovation and Technology in the Phosphate Industry - [SYMPHOS 2013] Anthraquinone and Alizarin forms and structures • Alizarin contains hydroquinone and catechol moiety (with two adjacent OH phenol groups); So, according to pH conditions, the catechol moiety could loose whether one proton or two protons HAP-ARS-MECHERRI 5 2nd International Symposium on Innovation and Technology in the Phosphate Industry - [SYMPHOS 2013] Assuming pK values (pK (2-OH) = 5.49 and pK(1-OH) = 10.85) of the diacid alizarin sulfonate given by Wu and Forsling, we have drawn the theoretical distribution diagram of alizarin sulfonate (ARS) and that of phosphate system. HAP-ARS-MECHERRI 6 2nd International Symposium on Innovation and Technology in the Phosphate Industry - [SYMPHOS 2013] Formation of Metal-ARS complexes via two modes: ¤ five-membered complex cycle: so-called « catecholate » or « diphenolate »; ¤ six-membered complex cycle: so-called « chelate » or « ketophenolate ». Translated from: Jana Sanyova, Contribution à l'étude de la structure et des propriétés des laques de garance, Thèse ULB, Bruxelles 2001. HAP-ARS-MECHERRI 7 2nd International Symposium on Innovation and Technology in the Phosphate Industry - [SYMPHOS 2013] Many authors like Moriguchi claimed two types of bonding of ARS could be observed on HA, one involving: • diphenolate or catecholate salt formation with 2 phenolic hydroxyl groups, • ketophenolate akin to chelate formation with a phenolic hydroxyl group and adjacent quinone oxygen Binding schemes according to Moriguchi et al., also reported by Ibsen and Birkedal HAP-ARS-MECHERRI The complexation mechanisms reported in the literature never involve participation of the sulfonate group (-SO3-) of the alizarin-sulfonate ARS. 8 2nd International Symposium on Innovation and Technology in the Phosphate Industry - [SYMPHOS 2013] Structures of Ca-ARS 1:1 and 1:2 complexes The doubly deprotonated ARS forms a 1 to 1 calcium complex. The monodeprotonated ARS forms a 1 to 2 complex. HAP-ARS-MECHERRI 9 2nd International Symposium on Innovation and Technology in the Phosphate Industry - [SYMPHOS 2013] The ARS dye changes its color with the variation of the pH from violet (for basic pH) to pink, red, orange and therefore to yellow (for the most acidic pH values). A very similar theoretical spectrum has been given by Turcanu and Bechtold for numerous pH values. Our experimental UV spectrum given for different pH values after addition of NaOH to 0.1mM ARS solutions. HAP-ARS-MECHERRI 10 2nd International Symposium on Innovation and Technology in the Phosphate Industry - [SYMPHOS 2013] Our experimental IR spectrum of HA with increasing concentrations of ARS [HA + (5-10-20)% of ARS]. At right: IR spectra of (a) HAP (microscopic method) and (b) ARS (KBr method) T. Moriguchi et al. / Journal of Colloid and Interface Science 260 (2003) 19–25. HAP-ARS-MECHERRI 11 2nd International Symposium on Innovation and Technology in the Phosphate Industry - [SYMPHOS 2013] pH jump: corresponds to a difference of pH for a given added volume of NaOH: HBO2 appears to be stronger in presence of polyols. pH shifts for some concentrations of alizarin during its titration with NaOH. The maximum pH shift occurs at 2 ARS for 1 Ca pH shift: corresponds to a difference of added volume of NaOH for a given constant pH value as a function of stoichiometry. HAP-ARS-MECHERRI 12 2nd International Symposium on Innovation and Technology in the Phosphate Industry - [SYMPHOS 2013] The maximum pH shift occurs for 2 ARS for 1 Ca The 1 to 2 calcium-alizarin complexforms as follows: Ca2+ + 2 ARS------ Ca(ARS)2 + 2 H+ HAP-ARS-MECHERRI Titration of 1.25 mm calcium ions in the presence of increasing ARS concentrations 13 2nd International Symposium on Innovation and Technology in the Phosphate Industry - [SYMPHOS 2013] The pH shift is lower than in the case of Ca + Ars with no phosphate, but occurs for 2 ARS for 1 PO4 Thus, Alizarin reacts with surface bound apatite calcium ions Titration of Ca/P solution with increasing amounts of alizarin We had : Ca/PO4= 1.5 = 3:2 => 2 Ca/2PO4=3 ; the maximum shift occurs for ARS/PO4 = 2 or 2 ARS/PO4 = 4 The complex formulae will be: (ARS)4Ca3(PO4)2. HAP-ARS-MECHERRI 14 2nd International Symposium on Innovation and Technology in the Phosphate Industry - [SYMPHOS 2013] pH jumps and pH shifts For the mixtures calcium-alizarin, calcium-phosphate and calcium-phosphate-alizarin, there are both a pH jump and a pH shift of the equivalent points to the titration of alizarin and of the phosphorous species originating from apatite HA. HAP-ARS-MECHERRI 15 2nd International Symposium on Innovation and Technology in the Phosphate Industry - [SYMPHOS 2013] Conclusion: We tried to show the interactions of calcium phosphate with alizarin dye model. But much remains to be done to elucidate the mechanisms of formation of many other metal-dye complex and especially for metal-phosphates-dye complexes encountered in industrial textile mixtures and waste residues. Pacific berber warrior double deprotonated Many thanks for your attention ! HAP-ARS-MECHERRI 16 Addendum: 2nd International Symposium on Innovation and Technology in the Phosphate Industry - [SYMPHOS 2013] Release of the potential acidity of (weak) boric acid via exaltation with polyols Pacific berber warrior double deprotonated pH jump during synergic titration of boric acid HBO2 0.1 M by NaOH 1M in presence of mannitol by the Sorensen method. HAP-ARS-MECHERRI 17 2nd International Symposium on Innovation and Technology in the Phosphate Industry - [SYMPHOS 2013] Addendum: Illustrative example of a synergic titration of copper ions with alizarin in presence of borates. Alizarin-borate complex structure as suggested by Panahi et al.