Selective ALD of TiO2 by the Reduction of Native Copper Oxide
advertisement

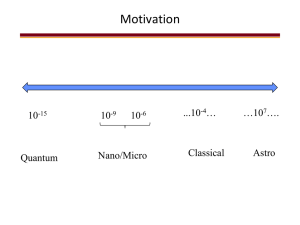
Selective Atomic Layer Deposition of TiO2 on Silicon/Copperpatterned Substrates UIC REU 2011 AMReL, University of Illinois at Chicago Abigail Jablansky Department of Chemical and Biomolecular Engineering, University of Pennsylvania What is ALD? • Atomic layer deposition • Method: – – – – Precursor (TDEAT) Purge (N2) Oxidant (H2O) Purge (N2) • Batch adsorption process • Easily controlled but time-consuming • Characterized with ellipsometry, X-ray photoelectron spectroscopy (XPS) • Diverse applicationswww.cambridgenanotech.com/ald Copper and Silicon • Conductive substrate • Small channels of conduction in microelectronics • Need a thin barrier layer on silicon • Copper oxidizes more easily – Selective ALD (SALD) – Native oxide www.electroiq.com • Prevention Native Oxides – Self-assembling molecules1 • Minimization – Limited air exposure2 – Few cycles3 • Reduction – GaAs oxide remains under HfO2 but converted under Al2O34 1Chen, Tao, Q.; Jursich, G.; Takoudis, C. App. Phys. Lett. 2010, 96, 192105 R.; Kim, H.; McIntyre, P.C.; Bent, S.F. Chem. Mater. 2005, 17, 536. 2Lee, H.D.; Feng, T.; Yu, L.; Mastrogiovanni, D.; Wan, A.; Gustafsson, T.; Garfunkel, E. App. Phys. Lett. 2009, 94, 222108. 3Tao, Q.; Overhage, K.; Jursich, G.; Takoudis, C. Submitted to Journal of Physi Chem. C. 2011. 4Frank, M.M.; Wilk, G.D.; Starodub, D.; Gustafsson, T.; Garfunkel, E.; Chabal, Y.J.; Grazul, J.; Muller, D.A. App. Phys. Lett. 2005, 86, 152904. Copper Oxides • Cu2O (cuprous oxide) – Linear – Most stable copper compounds at high T – Forms ammine under NH35 • CuO (cupric oxide) – Square planar – Decomposes at high T to Cu2O + O2 – H2 or CO reduction at 250oC5 • Cu2O forms first, then CuO if stable6 • Reduction methods 5Cotton, F.A.; Wilkinson, G. Advanced Inorganic Chemistry, 2nd ed. New York: Interscience Publishers, 1966, pp.894-902. 6Zhu, Y.; Mimura, K.; Lim, J.; Isshiki, M.; Jiang, Q. Metal. and Mineral Trans. A. 2006, 37A, 1231. Project Description • ALD of TiO2 onto Si/Cu wafers – Precursor: tetrakis(diethylamino)titanium (TDEAT) – Oxidizer: water • Compare 24-hr Cu (1 nm native oxide) exposure to 1-hr7 • Minimize exposure from reactor to ellipsometer, x-ray photoelectron spectroscopy (XPS) 7Tao, Q. PhD Dissertation, University of Illinois at Chicago, 2011. Reactor Schematic Ice bath Hot wall reactor Tao, Q. PhD Dissertation, University of Illinois at Chicago, 2011. Experimental Setup Characterization Ellipsometry • Reflects light off thin films • Measures polarization after reflection X-ray photoelectron spectroscopy (XPS) • X-rays are energy source • Measures kinetic energy, number of escaping electrons Results • Verified Tao’s work7 – Constant growth rate = linear growth Thickness of TiO2 on Si 35 30 Thickness (A) 25 y = 0.8587x R² = 0.9049 20 15 10 5 0 0 7Tao, 5 10 15 20 Number of cycles Q. PhD Dissertation, University of Illinois at Chicago, 2011. 25 30 35 Troubleshooting • Temperature – Increases along path to reactor – Keep oxidizer cold • Pressure – “Resting pressure” around 0.176 torr – Cycles during deposition • N2 tank, H2O level in bubbler • Check ellipsometer • Precursor level, clogged pipes Results (cont.) The colors could represent a deposition layer thickness profile or a chemical vapor deposition (CVD). Summary • Objective: SALD of TiO2 on Si for microelectronic applications • Method: reduce native oxide on Cu – Minimize air exposure (in progress) – In situ reduction (future work) • • • • Characterization: ellipsometry, XPS Results to date verify prior research Not enough data to conclude about TiO2 on copper Troubleshooting, design setbacks are important parts of engineering Acknowledgements • National Science Foundation, EEC-NSF Grant # 1062943 • CMMI-NSF Grant # 1134753 • Jorge I. Rossero A. • Runshen Xu • Arman Butt • Dr. Jursich • Dr. Takoudis References • Chen, R.; Kim, H.; McIntyre, P.C.; Bent, S.F. Chem. Mater. 2005, 17, 536. • Lee, H.D.; Feng, T.; Yu, L.; Mastrogiovanni, D.; Wan, A.; Gustafsson, T.; Garfunkel, E. App. Phys. Lett. 2009, 94, 222108. • Tao, Q.; Jursich, G.; Takoudis, C. App. Phys. Lett. 2010, 96, 192105 • Tao, Q.; Overhage, K.; Jursich, G.; Takoudis, C. Submitted to Journal of Phys. Chem. C. 2011. • Frank, M.M.; Wilk, G.D.; Starodub, D.; Gustafsson, T.; Garfunkel, E.; Chabal, Y.J.; Grazul, J.; Muller, D.A. App. Phys. Lett. 2005, 86, 152904. • Cotton, F.A.; Wilkinson, G. Advanced Inorganic Chemistry, 2nd ed. New York: Interscience Publishers, 1966, pp.894-902. • Zhu, Y.; Mimura, K.; Lim, J.; Isshiki, M.; Jiang, Q. Metal. and Mineral Trans. A. 2006, 37A, 1231. • Tao, Q. PhD Dissertation, University of Illinois at Chicago, 2011. • Falkenstein, Z.; Hakovirta, M.; Nastasi, M. Thin Solid Films. 2001, 381, 84. • Tompkins, H.G.; Allara, D.L. J. Colloid and Interface Science. 1974, 49, 410. • Sakata, Y.; Domen, K.; Maruya, K.-I.; Onishi, T. Appl. Spec. 1988, 42, 442.