ppt - Wits Structural Chemistry
advertisement
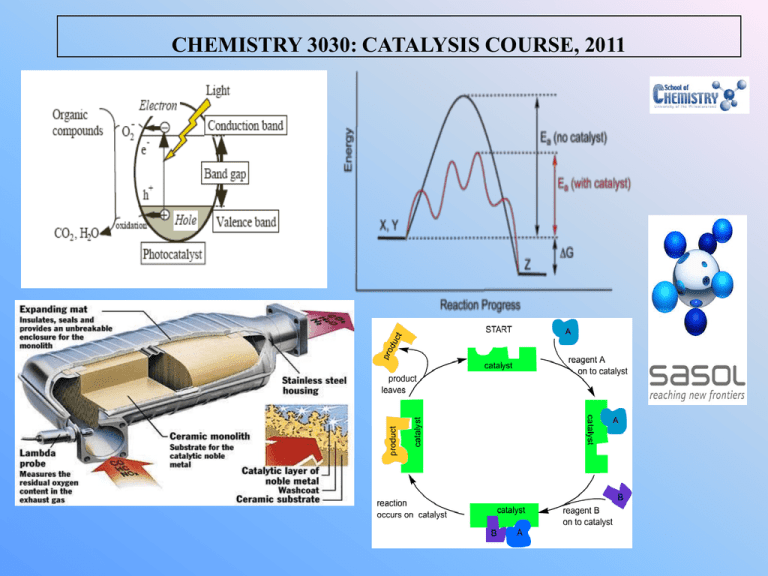
CHEMISTRY 3030: CATALYSIS COURSE, 2011
INTRODUCTION
WHAT IS THE DEFINITION OF A CATALYST?
Given the following reaction at equilibrium:
A2(g) +2B2(g) 2AB2(g)
Q:
What will happen to the equilibrium position in such a
reaction, if a small quantity of a catalyst were to be
added?
A:
2
INTRODUCTION
HENCE: A catalyst is any substance
(____________) which, when present in a
reaction mixture is directly involved with the
reaction sequence (mechanism), and that
increases the reaction rate (_________) without
altering the position of the thermodynamic
equilibrium, but is itself not consumed or altered.
WHAT THEN IS CATALYSIS?
A:
3
INTRODUCTION
HOW DOES A CATALYST DO THIS?
Reactants
Activation
Energy
Energy
Products
Reaction co-ordinate
Enthalpy
change
= uncatalysed reaction
4
THREE KEY ISSUES TO CONSIDER WHEN
DEVELOPING A CATALYST:
1. ACTIVITY (A)
This a measure of the _________ at which the
catalyst is able to transform reactants into
products. This speed is related to the rate
constant ‘k’ i.e. Rate = –k [reactants]n
The activity (A) of a catalyst is measured by the
SI unit: katal (abbreviated to kat).
If the activity of a catalyst is 1 kat : then it
‘enables’ the reaction rate to be___________
5
CONT.
Often it is necessary to disperse (scatter) the
catalyst (or ________) on a solid material which
has a high surface area. This material is called a
_________. Examples of supports are: Al2O3(s),
TiO2(s), CaCO3(s), carbon nanotubes (CNTs), etc.
In these cases, the _________ at which the
reactions of supported catalysts are measured,
have units of molecules converted/ surface area
of exposed active phase (in cm2).
If however the number of exposed catalyst sites
have been determined experimentally (for later)
then the rate has units of molecules converted/
exposed catalyst sites ____________________.
6
CONT.
2. SELECTIVITY (S)
Multiple products are often formed in a reaction
when a catalyst is added. The catalyst thus has an
activity for each reaction that leads to a different
product.
The catalyst selectivity is then just a ratio of the
activity of one product over another (more about
this later). The larger the ratio the higher the
catalyst selectivity for that product.
Alternatively selectivity can be viewed as the
ability of a catalyst to _____________ the rate of
_____________of the thermodynamically feasible
reactions more than the others.
7
CONT.
Consider the following reactions and then place
them in decreasing order of the catalyst
selectivity:
Cu/Zn/Al2O3
→
1) CO(g) + H2(g)
CH3OH(g) = 1.0 x 10-1 Kat
Cu/Zn/Al2O3
2) CO(g) + 2H2(g)
→
½C2H5OH(g) = 2.5 x 10-2 Kat
Cu/Zn/Al2O3
3) CO(g) + 3H2(g) →
CH4(g) + H2O(g) = 9.8 x 10-2 Kat
ORDER: _____________
8
CONT.
3. DEACTIVATION
For a variety of reasons catalysts can lose their
activity and hence their selectivity ___________.
Some reasons:
1) SINTERING (_______________)
Supported metal catalyst particles are oftentimes more
stable when they are ____________or spread out on
the support surface.
CoO nanoparticle
Carbon nanotube
9
CONT.
___________ occurs at high temperatures
when the supported metal catalyst particles
spontaneously migrate on the surface. They
combine/coalesce with one another to form
bigger particles. Hence the metal catalyst
________________.
A.Binder et al. J. Phys. Chem. C 2010, 114, 7816–7821 (Pd on SiO2 or TiO2)
10
CONT.
2) POISONING
Some elements/ions (e.g. Cl, S, C, etc.),
when they build up in concentration, can
block active sites on the surface of a
catalyst and hence reduce the activity and
selectivity of the catalyst.
For example: ___________, in leaded
petrol, can deactivate catalytic converters
in cars.
11
TYPES OF CATALYSIS
Several types of catalysis:
1) Homogeneous Catalysis
When the reactants and the catalyst are in
the ___________:
e.g. O3(g) + A·(g) → O2(g) + AO(g) ….(1)
AO(g) + O3(g) → A·(g) + O2(g) ….(2)
Q:
Which species is the catalyst and which
the intermediate?
12
TYPES OF CATALYSIS
2) Heterogeneous Catalysis
When the reactants and the catalyst are in
_________________:
For example: The photoreduction of carbon
dioxide on titania
Pt/TiO2(s)
CO2(g)
CO(g) + O2(g)
hν
13
Examples of heterogeneous catalysis:
1) CO + ½ H2 CO2 (Co, Co-Ni) - Fuel cells.
Selec Ox. of CO in H2
2) CH2CH2 + H2 CH3CH3 (Ni/Pd) Olefin
Hydrogenation – Fuel industry. c.a. 119 million
tonnes of C2H4 in 2010!)
3) CHCH + 2H2 CH3CH3 (Pt/Pd) Removal of
C2H2 from olefins by hydrogenation)
4) CO + H2 -CH2n+2- (Fe, Co) FischerTropsch. Fuels, waxes, etc.
5) N2 + 3H2 2NH3 (Fe, Ru) Haber Process.
Fertilizers, explosives..
6) C6H6 + 3H2 C6H12 (Cu -1925!, Ru,Ni) 90%
of cyclohexane used for Nylon 6 and 66.
14
TYPES OF CATALYSIS
3) Enzyme Catalysis
Enzymes are polymeric molecules which
regulate the majority of __________ reactions
that take place in living organisms. In the
main they are proteins which are made up of
amino acid building blocks or ____________.
Enzymes are ___________and have extremely
________________(typically between 10 to 103
molecules converted/enzyme/s).
15
CONT.
Q: How does an enzyme catalyse a reaction?
A: The reactant molecules that interact with an
enzyme are called ___________.
Each enzyme has a specific site (_________)
where only certain shaped substrates can fit
into or bind (______________). When the
substrate ___________binds to the active
site the enzyme changes shape
(___________) to form the ___________.
The reaction then takes place, product/s
formed and released, enzyme returns to its
original shape.
16
CONT.
http://www.mun.ca/biology/scarr/F09-20bsmc.jpg
17
TYPES OF CATALYSIS
4) Polymer Supported Catalysis
“These are catalyst systems comprising a polymer
support (often based on _________________in the
form of ___________to pack in a reactor) in which
catalytically active species are ___________through
chemical bonds or weaker interactions such as
hydrogen bonds or donor–acceptor interactions
and can be used repeatedly.”
PAC, 2004, 76, 889 (Definitions of terms relating to reactions of polymers and to functional polymeric materials (IUPAC
Recommendations 2003)) on page 896
18
CONT.
Example: Pd nanoparticles (PdNPs) immobilised on
microporous Poly(amidoamine) (PAMAM) dendrimers.
Shin Ogasawara and Shinji Kato J. AM. CHEM. SOC. 2010, 132, 4608–4613
19
CONT.
Suzuki-Miyaura reaction in water.
Advantages:
• Water as a solvent for
organic rxn! GREEN rxn!
•In bio-active compounds
synthesized the catalyst
is easily retrievable : No
___________ of the
product!
•Reduced costs (Pd=$$$);
catalyst ___________
Shin Ogasawara and Shinji Kato *4608 9 J. AM. CHEM. SOC. 2010, 132, 4608–4613
20
CLASSIFICATION OF HETEROGENEOUS
CATALYSTS (BOND pg10)
Catalyst Type:
Examples:
Reactions:
Metals
Ni, Pd, Fe, Pt, Ag
Hydrogenation
Dehydrogenation
Hydrogenolysis
Oxidation
Semiconducting
oxides
/sulphides
NiO, ZnO, MnO2,
Dehydrogenation
Cr2O3, Bi2O3-MoO3, Desulphurisation
WS2
Oxidation
Hydrogenation
21
CLASSIFICATION OF HETEROGENEOUS
CATALYSTS (BOND pg10)
Catalyst Type:
Examples:
Reactions:
Insulating oxides Al2O3, SiO2, MgO
Dehydration
Acids
Polymerisation,
Isomerisation,
cracking,
alkylation
SiO2-Al2O3,
Zeolites
22
Catalysts and Surfaces
A reactant must react with the surface atoms of a
catalyst. Hence the more atoms on the surface, the
more reactants can be transformed into products.
Thus the expectation that high surface areas lead to
___________.
Surface Areas fall into three categories:
1. 10 m2 g-1 Small (e.g. _______________)
2. 200 m2 g-1 Normal (e.g. _______________)
3. 1200 m2 g-1 Large (e.g. _______________)
23
Catalysts and Surfaces
The rate might be expected to be __________ to the
number of surface atoms BUT not all surface atoms
are the _______. This gives rise to the concept of an
_____________(Taylor 1925).
Q: How then can the surface area be maximized?
A: _______________
For spherical particles on a hemispherical support,
the total surface area is give by
SA (in m2)= ____________________
Where M = total mass of catalyst, = density and
r = average particle radius, Vpart= volume of particle.
24
Catalysts and Surfaces
Q:
A batch of hemispherical catalysts (support
and active nanoparticles) weighs 1.23 g
and has a density of 3.14 g/ml. What is the
total surface area of the catalysts if they
are loaded with spherical nanoparticles
with diameters of 50 nm?
A: SA = _______________
25
Q: How then can the surface area be maximized?
A2: __________
Factor:
Effect of
Temperature
Heat
transfer
Chemical
reactivity
Inorganic
support
Organic
support
Good
_______
Good
_______
OK
_______
26
Q: How then can the surface area be maximized?
A2: __________
Promoters are substances that increase the _______
_______, even though they are not catalysts by
themselves. In addition they “allow the active phase to
function at its _______________” (Bond pp 76)
e.g. Co or Ni in WS2 catalyst for desulphurisation†
† C. Roukoss et al. / C. R. Chimie 12 (2009) 683-691
There are two types of promoters
1. _________
2. _________
27
Types of promoters:
1. Structural
A structural promoter ___________by separating
the surface ___________.
For e.g. The active phase for the NH3 synthesis
catalyst is Fe, but its promoters may include:
_________, ________, _____ and ______*. These
inhibit Fe crystallites from coalescing.
*I. Siminiceanu, I. Lazau, Z. Ecsedi, L. Lupa*, C. Burciag.Chem. Bull.
"POLITEHNICA" Univ. (Timisoara) Volume 53(67), 1-2, 2008.
28
Types of promoters:
2. Electronic
Electronic promoters are effective due to their
______________. The most widely used electronic
promoters belong to _______, _______ and the
_______.
For Groups 1A and 2A, their ability to promote is
inversely proportional to their electronegativity.
Examples: Cs > K > Na (Group 1A)+
Ba > Ca > Mg (Group 2A)++
+ S. Murata, K. Aika, T. Onishi, Chem. Lett., 1990, p. 1067.
++ S. R.Tennison, in: J.R. Jennings (Ed.), Catalytic Ammonia Synthesis, Fundamentals and Practice, Plenum
Press, New York, 1991, p. 303.
29
Electronic and structural promoter?
When Cs is added:
•No Caesium ruthenates form.
•The electronic properties change.
•RuO2 clusters reduced to metallic Ru. Increased Activ.
Y.V. Larichev. Effect of Cs+ Promoter in Ru/MgO Catalysts.
J. Phys. Chem. C 2011, 115, 631–635
30
Problem
Given the following data for the lanthanides,
place them in order of increasing ability to act
as electronic promoters:
Element
Pauling’s Electronegativity value
Samarium
1.198
Lutetium
1.201
Lanthanum
1.101
______________
31
Q: How then can the surface area be maximized?
A3: ______/ _______ maximization
Sol-gel process
TEOS = Tetraethyl
orthsilicate
Polymers include: polyvinyl
alcohol and polyethylene
glycol with different
molec.masses
S SATO, T MURAKATA, T SUZUKI,
T OHGAWARA. JOURNAL OF MATERIALS SCIENCE
25 (1990) 4880-4885
32
Q: How then can the surface area be maximized?
A3: _______/ _______maximization
No polymer
Polymer of low
molec. mass
Polymer of
higher molec.
mass
Mean pore sizes increase
from 3 nm to 7 nm
S SATO, T MURAKATA, T SUZUKI,
T OHGAWARA. JOURNAL OF MATERIALS SCIENCE
25 (1990) 4880-4885
33
ATOM SURFACE CONCENTRATION
The atom surface concentration can be
determined by the _____________
Assume that the bulk density is 1 g/cm3
then the molecular density will be 5 x 1022
molecules per cm3. The surface
concentration (molecules per cm2) is
proportional to _______if one assumes
cube like packing. This gives a value of
_______molecules per cm2.
34
DISPERSION
The fraction of the atoms on the surface is
referred to as _______. Mathematically,
dispersion (D) is the ratio of the number
of surface atoms (NS) to the TOTAL no. of
atoms (NT): i.e. _______
For very small particles D = 1
However, as the particle grows the
number of surface atoms will _______.
For a cube of 100 Å, D = 10-3 !!!!
35
DISPERSION
Dispersion (D)
1.0
0
1000
2000
Total number of atoms
3000
36
Surface Atoms
Consider a cube of metal (or metal oxide). The
surface atoms rest on the bulk atoms and so must
reflect this situation. Previously you learned about
how atoms can pack and the way in which
structures were built up. Example: _______
http://www.ndt-ed.org/EducationResources/CommunityCollege/Materials/Structure/
metallic_structures.htm
37
Surface Atoms
Consider a cube of atoms with the _____structure. We
can take slices through this structure and this will
yield different faces with Miller Indices: (100), (111),
(100), etc.
http://www.diracdelta.co.uk/science/source/m/i/miller%20indices/image001.gif
M Bowker, The Basis and Applications of Heterogeneous
Catalysis, Oxford University Press, 1998., pp 12.
38
Surface Atoms
It is quite difficult to get surfaces with only one type
of face. Most surfaces have many faces and contain
_______, _______, _______ and _______. Thus
surfaces are generally not _______. This has
implications for the reactant molecules (see
example with ammonia synthesis that follows).
Different arrangements of surface atoms have
different _______ _______. Generally, surfaces
with _______ coordination number have the
_______ surface free energy (are the most
reactive).
39
Adatom
Step
Kink
edge
Terrace
A. N. Chaika, S. I. Bozhko, A. M. Ionov,
A. N. Myagkov, and N. V. Abrosimov.
Semiconductors, 2007, Vol. 41, No. 4,
pp. 431–435
http://www.oup.com/uk/orc/bin/9780199236
176/lecturer/figures/ch26f19.jpg
40
Spectroscopy in Catalysis: An Introduction, Third Edition, J. W. Niemantsverdriet
41
Copyright 8 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, ISBN: 978-3-527-31651-9
Atom type
Adatom
Kink edge
Step
Terrace
Top plane co-ordination
0
3
4
6
Thus reactivity order is: _______ > _______
> _______ > _______
M Bowker, The Basis and Applications of Heterogeneous Catalysis, Oxford University Press, 1998., pp 13.
42
Single crystal faces of Fe for NH3 synthesis
G.A. Somorjai, N. Materer / Surface structures in ammonia synthesis. Topics in Catalysis 1 (1994) 215-231
43
Molecular Heterogeneous Catalysis
Metal cluster chemistry can assist as an important
model to illustrate the interconnectedness between
_______ chemistry and _______ chemistry.
Consider the _____________ reaction of formic acid
catalyzed on different metal surfaces:
H-COO-H (aq)
CO2(g) + H2(g)
Metal
Plotting the _______ of each metal surface versus the
standard enthalpy of formation (ΔHof) of each metal
formate, gives what is called a ‘______________’ :
44
Molecular Heterogeneous Catalysis
_______
______________
Speed of
formation of
surface
intermediate
is low.
_____________
_____________
Speed of
decomposition
of surface
intermediate
is low
http://www.oup.com/uk/orc/bin/9780199236176/lecturer/figures/ch26f19.jpg
45
Molecular Heterogeneous Catalysis
The shape of the ‘_______’ plot is consistent with the
notion that surface intermediates closely resemble
bulk intermediates (formates in this case).
This is based on the _______ _______. This implies
that:
Reaction rate = f(_______, _______).
Thus the optimum catalytic performance does not
relate to a specific _______ _______ but to a balance
of interaction & desorption. These are the elementary
steps in _______ _______.
46
Molecular Heterogeneous Catalysis
Wolfgang Sachtler showed that when reacting
molecules _______ onto a surface they form _______
_______. Furthermore, these complexes result in the
partial destruction of metal-metal bonds and lead to a
‘______________’ of the surface. This was later
termed ‘_______ _______’.
Somorjai & Muetterties showed that the _______
_______ in a catalytic reaction, as well as the surface
complexes are similar to homogeneous _______
complexes and reactions.
47
Molecular Heterogeneous Catalysis
Comparison of some Somorjai &Muetterties surface
complexes with known organic complexes:
48
ADSORPTION
Molecular and/or atomic species have essentially two
ways in which they can attach or adsorb onto a
surface: _______ or _______.
1. Physical Adsorption or ___________
Physisorption often occurs in any liquid/solid or
gas/solid system where the molecular/ atomic
species attach to the solid surface through _______
_______ _______ (van der Waals forces).
The elementary step in physisorption from a gas
phase does not involve an _______ _______.
49
1. Physisorption (cont.)
The typical binding energy of these physisorbed
species on a surface is between ___________. (No
chemical specificity).
The process is _______ _______ _______, with
little energy. See example of helium gas on metal
surfaces afterwards.
The adsorption enthalpy can range between
_______ and _______.
For physisorption, under appropriate conditions,
gas phase molecules can form _______ adsorption.
50
Example: The physisorption profiles of He on various
metal surfaces.
E. Zaremba and W. Kohn (1977). "Theory of helium adsorption on simple and noble-metal surfaces". Phys.
Rev. B 15 (4): 1769. doi:10.1103/PhysRevB.15.1769.
Retrieved from "http://en.wikipedia.org/wiki/Physisorption"
51
2. Chemical Adsorption or _____________
Chemisorption adsorption occurs when
molecular/atomic species chemically attach to a
surface through _______ _______. New species are
formed. _______ _______ _______ _______
The typical binding energy of chemisorbed species
on a surface is between _______. Binding is
usually chemically specific.
The process is less easy to reverse and often takes
a lot of energy to do so. Sometimes the process is
_______ i.e. due to _______ of the species.
Example: _______ _______ _______ _______
_______ _______ _______ _______ _______.
52
2. Chemisorption (cont.)
The elementary step in a chemisorption process
from the gas phase often involves an _______
_______. (Recall dissociation of oxygen on metal.)
In chemisorption, because the molecular/atomic
species are adsorbed on the surface by covalent
bonds, they often only form a single or _______
adsorption.
In chemisorption, the adsorption enthalpy can
range between _______ and _______.
Q: Why are the adsorption enthalpies of physisorption
and chemisorption negative?
53
ADSORPTION ISOTHERMS
Q: Why is it important to obtain a
relationship between the quantity of a
substance adsorbed on the surface of a
solid and its gas phase pressure?
1. The _______ of gas coverage on the
surface
can be _______ determined.
1. The _______ of the adsorption of the
molecules
can
be
quantitatively
determined (i.e. chemi or physisorption).
2. The _______ _______ of the solid can be
quantitatively determined.
3. The _______ of the catalytic system can
be modelled.
54
ADSORPTION ISOTHERMS
Q: What then is an adsorption isotherm?
A: “The relationship between the _______ of
gas adsorbed on a surface and the
_______ with which it is in equilibrium, at
a
_______
_______,
is
called
an
adsorption isotherm” (Bond pg 15).
Suppose the maximum surface that
could be covered was ____ and that it was
covered by an amount __, then the ratio or
fraction (___) that the surface is covered
is: _______ _______, then an adsorption
isotherm, based on various assumptions
can be developed.
55
LANGMUIR ISOTHERM
Suppose that a solid surface has a gas (of a
certain pressure) _______ _______ on its
surface and at dynamic equilibrium then:
3
1
1
2
2
3
56
If the ratio or fraction that the surface is
covered is θ, then Langmuir made the
following assumptions:
1.
Adsorption isotherms do not exceed a
_______ _______. Thus
molecular/
atomic species can only maximally fill the
surface, then no further adsorption
occurs i.e. _______ _______ _______
2.
All sites on the surface are _______ and
_______. This implies that the surface is
_______ _______ and that the _______
_______ would be equivalent
throughout the surface.
57
3.
When molecules adsorb onto a surface,
they _______ _______ or _______ one
another or incoming molecules that will
adsorb. i.e. molecules will adsorb onto a
site ___________ from whether or not a
site next to them is _______ or _______.
In the light of what you have already
learned, take some time to critically
analyze Langmuir’s assumptions.
58
DERRIVATION OF LANGMUIR ISOTHERM
1) Mono/single site adsorption
Consider a gas A that can reversibly _______
and ______ on an active site *, then:
Where kA = ________________________
Where kD = ________________________
the number of molecules colliding with the
surface in unit time is proportional to the
____________of gas A i.e. ____________
59
Suppose the surface has a total of _____
________and at some time the ________
________ is , then:
Fraction of unoccupied sites = ________
Number of unoccupied sites = ________
Rate of adsorption PA
But:
Rate of adsorption no of unoccupied sites
________
So:
Rate of adsorption PA × N(1-)
Rate of adsorption = _____________,
(where kA = _____________________)
60
But, the rate of desorption amount of
adsorbed gas
________
Rate of desorption = ________
(where kD = _____________________)
At equilibrium:
Rate ___________ = Rate ___________
thus: kA× PA × N(1-) = kD × N
Where___ and ___ are the equilibrium values
of pressure and surface coverage.
61
Cancelling like terms and rearranging gives:
(kA× PA ) – ( × kA× PA ) = kD ×
So:
= (kA× PA ) (kD + kA× PA )
Then let _______ or the ______________
_________ then you obtain the following:
L
___
This is the ______________for _____ site
adsorption.
62
Thus:
________
________
________
Recall:
b = kA/kD
________
Langmuir adsorption isotherm
63
If V = volume actually covered
And Vm = monolayer coverage then:
θ = ________ then since
___
Then rearranging and substituting gives:
This is in the form Y= mX + C , where Y = ____
m = ____ , X = __ C = ____.This is allows the
monolayer coverage to be calculated
64
Since ________
and kA = AA×e–{EA/RT} with kD = AD×e–{ED/RT}
____
Where ________
Thus b is a function of ________ and
________ at temperature ________.
When b is ________ then ________ is _______
bonded, conversely when b is ________ it is
________ bonded.
65
2) Dual site adsorption
Consider a gas A that must strike the surface
at a location where there are _____ adjacent
active sites *, then:
Up to fairly high fractional coverage () it can
be assumed that the adsorption of _____
_________ will depend on the fraction of
vacant sites or ____, and that the adsorption
of the other fragment will also depend on
this ____________.
66
2) Dual site adsorption (cont.)
Then: Rate of adsorption = _____________
Rate of desorption = ________
At equilibrium: Rate of ads= Rate of desorp,
So: k’APA[N(1 - )]2 = k’D(N)2
If b = k’A/k’D, Then by subsitution and
Rearrangement: = (bPA)½ /(1 + (bPA)½ )
For the monolayer coverage (Vm), again, let
= V/Vm, then
This is in the form Y= mX + C , where
Y =______, m = ____ , X = ___ C = _______.
Thus a plot of _______ against ___ will give a
straight line.
67
Measured amounts adsorbed of the pure gases CH4 ( ),
CO2 ( ), and N2 ( ) on AC Norit R1 at T D 298 K. Simultaneous
fit of all data with the generalized dual-site Langmuir isotherm (—)
F. DREISBACH, R. STAUDT AND J.U. KELLER Adsorption 5, 215–227 (1999)
68
3) Non-competitive adsorption
It is possible that ___________(gas A, and
gas B), may be in the same container and
will adsorb on different sites. The adsorption
is _________________.
Then the isotherm for each gas is simply a
Langmuir isotherm for ________ gas. i.e.
= bPA/(1 + bPA) for gas A, and
= bPB/(1 + bPB) for gas B
69
4) Adsorption of more than one species on the
same surface
Consider the reaction of _______ gases on a
surface (i.e. the adsorption is ____________).
with rate constants ka(A) and kd(A)
with rate constants ka(B) and kd(B)
Then
A = bAPA/(1 + bAPA + bBPB)
And B = bBPB/(1 + bAPA + bBPB)
70
5) The General expression
We can work with 1,2,3,4, ….. up to i gases.
Each gas can be expressed by a Langmuir
Isotherm:
A = bAPA/(1 +biPi)
Other Non-Langmuir Isotherms
i) ____________ Isotherm
This assumes that a __________ decrease of
the enthalpy of ____________ occurs with
fractional coverage. ( = kP1/n where k and n
are constants with n > 1)
71
L Zhang, S Hong, J He, F Gan, Y-S Ho. Clean – Soil, Air, Water 2010, 38 (9), 831–836.
Freundlich isotherms obtained using linear and nonlinear regression methods for the
adsorption of phosphorus onto Al2O3 at temperature of 308 K.
72
ii) __________ Isotherm
Assumption here is that the ________ heat of
adsorption falls off _________ with coverage:
θ= k’ln(k”bPA)
where b and PA have been defined previously.
73
SUMMARY OF LANGMUIR ISOTHERMS
74
POROUS MATERIALS
Many materials are _______. Information about
the pore ______, pore ________, pore ________
as well as the _________ can be obtained from
two different types of adsorption experiments:
1) Multilayer gas adsorption
Here we use an _________ _________ or
_______e.g. N2, Ar, Kr to physisorb onto the
material.
2) Mercury Porosimetry
Liquid mercury is forced _______ into the pores
of the material (e.g. _______ gives information
on ______pore radius)
75
PORE SHAPES/TYPES
Uniform/cylindrical Blind pore
Through pore
Funnel shaped
Ink bottle shaped
Closed pore
Porous
network
76
POROUS MATERIALS
Pore Size
Pores are classified according to size
_________ < 2 nm
_________ 2 nm < x < 20 nm
_________ > 20 nm
Microprous starch
Yun Wu, Xianfeng Du, Honghua Ge and Zhen Lv Starch/
Stärke 2011, 00, 1–9 DOI 10.1002/star.201000036
77
IUPAC Classification
of porous solids
There are _________ of adsorption
isotherms that have been observed.
Each gives information about the
types of pores contained in a solid
as well as the capacity of the solid to
adsorb a gas.
78
IUPAC Classification of porous solids
I
II
III
IV
V
VI
79
IUPAC Classification of porous solids (Cont.)
In the previous figures the _________ of the
curve gives information about the solid – its
_________. Let us examine these a bit closer:
Microporous solids (see I)
At ______pressure: adsorption in _________ first
At _________ pressure: then coverage of
_________ surface takes place.
Mesoporous solids (see IV)
At _____ pressure: ________coverage (plateaus)
At _________ pressure: adsorption in _________.
After the pores are filled adsorption occurs on
the external surface.
80
Macroporous solids (see II)
At _____ pressure: __________ coverage
At _______ pressure: _______ coverage until
condensation occurs. There tends to be
overlap between the 2 regions
Uniform ultra-microporous solids (see VI)
If all sites the same: _________ coverage
If not: _________ isotherm for _________
Example: Zeolites
Range of pore sizes
Zeolite (small size
range)
81
HYSTERESIS LOOPS
Evaporation from a pore takes place at a _________ than
condensation thus the path of _________ differs from
_________. Four types of Hystereses have been identified
and classified (IUPAC)
0
Type H3
0.25
0.5
0.75
1.0
0
0.25
0.5
0.75
1.0
0
Adsorbed volume
Type H4
Adsorbed volume
Adsorbed volume
Type H2
Adsorbed volume
Type H1
0.25
0.5
0.75
1.0
0
0.25
0.5
0.75
P/P*
P/P*
P/P*
P/P*
The four _________ shapes of adsorption isotherms
82
typically associated with N2 adsorption
1.0
HYSTERESIS LOOPS (cont.)
H1/H2 TYPE
Particles with _________ or aggregates of
_________ particles
H1: uniform size/shape
H2: non-uniform size/shape i.e. different size pore
mouth and pore body e.g. ink bottle type pores
H3/H4 TYPE
Aggregates with _________ pores
H4: uniform size/shape
H3: non-uniform size/shape
e.g. zeolites, carbons
83
KELVIN EQUATION
Lord Kelvin noted that the evaporation of
condensed gas molecules from a surface with very
fine pores is more difficult than their condensation.
This is because there is a greater probability, as
compared to a _________ _________, that the
molecules which evaporate from a ____________
meniscus will _________.
Using the Kelvin equation, it is possible to
measure a pore radius at a given P/P*:
_________ _________, where
V = molar volume of liquid, = surface tension
r = pore radius, = contact angle (usually = zero)
84
R=gas constant, T = temperature
10.0
20.0
30.0
Consider a zeolite material onto which
nitrogen was adsorbed and desorbed.
If the P/P* was 0.25 at –183.15oC and the
surface tension of nitrogen was
1.0 x 10-1 Nm-1 (and the contact angle
was zero), then use the graph to
calculate what the pore radius of the
zeolite was.
0
Adsorbed volume ml/mol
PROBLEM
0
0.25
0.5
0.75
1.0
P/P*
85
SURFACE AREA
Porous Materials
I) Internal surface area
If the pores are _______, _________ uniform
cylinders then: S = 2Vp/r
Vp =pore volume
r = radius of pore, S = internal surface area
II) Total Surface Area
This is given by: S = nmLm, where:
nm = moles gas adsorbed in __________
m = area of ________ adsorbed molecule
L (or N) = Avagadro constant
86
SURFACE AREA
The Brunauer, Emmett and Teller (BET) Method
(Used on type II adsorption curves for multiple layer
physisorption - see IUPAC classification of porous solids)
Here:
V =θ=
CP
Vm
(P* –P) 1 + (C–1)P
P*
Which can be rearranged to give the ____________:
P
P*
Vm 1 – P
P*
Y
=
1
C.Vm
P
+ (C-1) P*
C. Vm
X
Slope
Y – intercept. Thus calculate Vm
Vm = volume of gas, monolayer coverage,
C = _________ _________ constant
87
12
8
4
0
P/P*
Vm (1-P/P*)
/ 10-1 cm-3
16
20
_________ _________ _________
0
0.1
0.2
0.3
P/P*
0.4
0.5
88
BET SURFACE AREA (cont).
Single point method
This is a _________ method. It arises because the
slope > intercept (which tends to zero when P/P* is in
the 0.2 to 0.3 region see (type II)).
Hence assume the BET plot passes through the
origin. i.e. assume Y-intercept at the origin.
Then slope = (C-1)/C.Vm and thus Vm can be
calculated.
An error bar of 5% is acceptable in these
experiments.
89
Single point method (cont.)
Alternatively, if a simple extrapolation is made from
the _________ of a set of data, then the molar
volume can be obtained. This method delivers a
rough estimate of the molar volume of within 10%
(Bowker pp 57).
V
P/P*
90
BET SURFACE AREA (cont).
The constant C in the BET equation is related to the
following equation:
C = e[(Ha – H1)]/RT, where Ha = enthalpy of _________ of
the _________ and H1 = enthalpy liberated from the
second and subsequent layers (similar to the
_________ of the gas). Thus C helps give an
estimate of ΔHads and it influences the ______ of the
adsorption isotherm: C=10 000
V
C=10
C=2
C=1
0
P/P*
1
91
SURFACE AREA
Gases used for analyses:
Gas
N2
Ar
O2
Area/10-20m2
16.2
13.8
14.3
Saturation P (torr)
760 at 77K
220 at 77K
760 at 77 K
There is a limit on using nitrogen: _______ pore
corresponds to 5 molecule width. Hence we use
Ar or Kr for low surface area measurements
(_________ ).
92
KINETICS
Kinetic measurements and the interpretation of the
kinetic data lies at the heart of catalysis. Kinetic
analysis allows for:
Reactor design
Correlating and rationalising catalytic activity
Mechanism determination
Rates of reaction, Order, Effect of Temperature
Consider the reaction
__A +
__B
__C
Rate (r) given by (rate of form = rate of consump):
r = – (1/__)dA/dt = – (1/__)dB/dt = +(1/__)dC/dt
Units of r = _________
93
KINETICS
In a heterogeneous reaction the rate will depend
on the _____________area available to the
reactants. This is expressed as the ____________
(TOF). TOF = no of molecules converted per unit
of time _______________ .
Gas Phase Reaction (Homogeneous reactions)
We know that the reaction rate can be expressed
in terms of _________ _________ .
Reaction : aA +
bB
cC
Rate = k(PA)a(PB)b(PC)c ….This is referred to as
a ________________
94
Given: Rate = k(PA)a(PB)b(PC)c ,
a,b,c = orders of the reactants (don’t have to =
integers).
k = rate constant
Rate can be expressed per _________ :
r = mrm, where m = mass of catalyst OR
If the total surface area (S) of the catalyst has
been determined, then rate can be expressed
______________ : r = Srs
Recall the Arrhenius equation:
k = Acatexp(–Ecat/RT)
Where Ecat = Activation Energy
And Acat = pre-exponential factor
95
ADSORPTION MECHANISMS
We will now look at two different mechanisms
proposed for adsorption:
(A) Langmuir-Hinshelwood
(B) Eley-Rideal
96
I) LANGMUIR-HINSHELWOOD (L-H) MODEL
Three general assumptions to this model:
1. Adsorption is _____ and _________from the
gas phase
2. The reaction of the adsorbed molecules is the
rate determining step (RDS) i.e. the surface
chemical reaction = RDS i.e. k very small
3. The _________ of an _________ species is
determined by the appropriate Langmuir
Isotherm.
Two types to consider:
97
(A) L-H model for unimolecular reactions
Example: E(g) E (Ads) → C(g)
Molecule E
at PE E
C
Product
Molecule C
Surface rxn
θE E =RDS C
dPE
Rate
k E
dt
Rate
dPE
dt
dPC
dt
kbE PE
(1 b E PE )
If bE or PE are _______, then: Rate= k bE PE …i.e. __________
If bE or PE are _______, then: Rate → k …..i.e. ____________
Things to note about L-H model for
unimolecular reactions
1. This type of kinetics is not specific
to catalysis
2. Mainly applies in:
3 step reactions
Pre-equilibrium systems
e.g. Michaelis Menton equation
for enzymatic catalysis in
Biochemistry
99
(B) L-H model for bimolecular reactions
A(g)
A (Ads)
And B(g)
B (Ads)
Surface rxn
Fast
A(Ads) + B(Ads) → AB(Ads) → AB(g)
=RDS
Two extra assumptions to this model:
1. Molecules A and B are adsorbed on _______
sites with _____________
2. Product molecule AB is very ___________and
comes off the surface fast.
Product molecule BA
Molecule B
at PB
Molecule A
at PA
θB
θA
RDS
b B PB
B
(1 b P b P )
A
Rate
A
B
bA PA
A
(1 b P b P )
B
A
kbB PB b A PA
(1 b A PA bB PB )
Rate k B A
2
A
B
B
.. Langmuir-Hinshelwood
Equation
Things to note about L-H model for
bimolecular reactions
1. If molecules A and B are _______ adsorbed,
bA and bB are _______ , then:
Rate = k’ PAPB ….i.e. _______,
(Where k’= kbA bB)
2. If molecule ____is _______ adsorbed and
molecule B is strongly adsorbed i.e.
_____________then: Rate = k’’ PA/ PB ,
(Where k’’= kbA/bB). This is an indication that
B poisons the surface.
3. Similarly the rate is affected if the _______
_______of molecule A and B are varied:
102
The effect of surface concentration on the
rate in bimolecular reactions
Rate limited
by surface
Concentration
of A
rate
For constant PB
Rate limited by
surface concentration
of B
PA
θB>>θA
θA >> θB
II) Eley-Rideal (E-R) model for bimolecular reactions
Unlike the L-H model, in the E-R model it is
proposed that an adsorbed molecule may react
_______with an _______ gas molecule by a
collisional mechanism. The surface reaction is
still the RDS i.e.
PA
PB
θA
RDS
Eley-Rideal bimolecular surface reactions
Rate k A P B
_________
_________
bAPA << 1
Thus:
Rate = k bA
θA = 1
Rate
Note: For
constant PA,
the rate is
always first
order wrt PB
kb A PB P A
(1 b A P A )
_________
_________
For constant PB
bAPA >> 1
Thus:
Rate = k PB …zero
PA
order
PA PB …….. first order in A
in A
_____
_____
How can the two bimolecular reaction models
be distinguished from one another?
Experimentally if the reaction rate is measured as a
function of the surface coverage of A i.e. θA, then the rate
will initially increase for both mechanisms at
___________________. There will be slight to no difference
between the two proposed models observed. BUT:
If the reaction proceeds by the model proposed by EleyRideal then at ___________________(i.e. θA 1) the
_________________until the surface is covered by A.
If the reaction proceeds by the model proposed by
Langmuir-Hinshelwood then the ______________
________________and then gradually decreases to zero as
θA 1.
The effect of surface coverage on rate for the
Eley-Rideal and Langmuir-Hinshelwood models
Rate
Q: Will the rate of E-L
model increase ad
infinitum?
0
θA
1
THE EFFECT OF TEMPERATURE ON KINETICS:
CURVATURE IN ARRHENIUS PLOTS
In heterogeneous systems we will still assume for the
reaction:
A
C
Rate = –dPA/dt = kA = kbAPA.
BUT in this equation BOTH ___ and _____ are functions of
temperature. For instance:
1. If bA is ________ and _____ is ________, then k will be the
only variable that is a function of T. i.e. k = A·exp(Etrue/RT)
2. If bA is small then tends to _____, then from the van’t
Hoff Isochore we have:
(dlnbA)/dT = HθA/RT2 , then ∫(dlnbA) = ∫ (HθA/RT2) dT
108
Thus:
lnbA = –HθA /RT + C OR bA = C·exp(–HθA/RT)
(where C = integration constant & –HθA is the standard
molar enthalpy of adsorption of reactant A)
Now we have rate = kA and A = bAPA
We can substitute into the equation for both k and bA
i.e. Rate = –dPA/dt = kA = kbAPA = k[C·exp(–HθA/RT)](PA)
= A·exp(–Etrue/RT)[C·exp(–HθA/RT)](PA)
Re-writing this out:
= PA·A·C·exp(–Etrue–HθA)/RT
= PA·A·C·exp(–Eapp)/RT
Where: (–Eapp) = (–Etrue–HθA), hence Etrue = Eapp–Hθa
and since HθA is always negative, then Etrue > Eapp
109
Arrhenius plot for a catalysed reaction over a range of
temperatures gives:
I
Log 10 Rate
III
II
1/T
II
III
Surface coverage 1
1>>0
~0
Order,n
0
1>n>0
1
Slope x 2.3R
Etrue
----
Eapp
I
110
THE EFFECT OF DIFFUSION ON KINETICS
Diffusion or mass-transport limited reactions are
those in which the transport of the ________ /or
____________ the catalyst influence the rate:
1.
2.
3.
4.
Rate [catalyst]n and n< 1 vs ______________
Rate stirring rate vs ____________________
Eact about 10 – 15 kJ mol–1 vs ______________
Rate (temperature)1/2 vs _________________
An Arrhenius plot showing the onset of
diffusion limitation can be drawn:
111
Log 10 Rate
R’
A’A: Surface reaction is
rate-limiting
Q’
A
Q
R
B’B: Reaction
is diffusion
limited
1/T
____________ for diffusion limited reactions the RAQ’ curve
(purple curve) is detected, because the slower of the two
processes controls the rate.
112