Topic 18. Acids and bases
advertisement

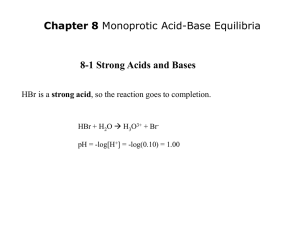
Topic 18- Acids and bases 18.1 Calculations involving acids and bases 18.2 Buffer solutions 18.3 Salt hydrolysis 18.4 Acid-base titrations 18.5 Indicators The relation between oxonium ions and hydroxide ions 18.1 Calculations involving acids and bases • pH = -log[H3O+] pOH = -log[OH-]] • [H3O+] = 10-pH [OH-] = 10-pOH • pH + pOH = 14 [H+]=[H3O+] Calculate the pH in following solutions: • 1M HCl pH = -log[1] = 0 • 0.001 M HNO3 pH = -log [0.001] = 3 • 0.5 M H2SO4 2H+ pH = -log [2*0.5]= 0 • 0.15 M NaOH pOH =-log[0.15]= 0.82 pH = 14-0.82 = 13.18 Calculate the [H3O+] in following solutions • pH = 5,5 [H3O+] = 10-5.5 = 3.2*10-6 • pH =- 1 [H3O+] =10-(-1) = 101 = 10 M Autoprotolysis of water H2O + H2O H3O+ + OH- Kc= [H3O+] . [OH- ] . [H2O] [H2O] The concentration of water is not changing- it is constant Kw= K . [H2O] . [H2O] = [H3O+] . [OH-] Kw = the dissociation constant of water Kw = dissociation constant of water H2O + H2O H3O+ + OHIn 25oC pure water: [H3O+] = 10-7 mol/dm3 [OH-] = 10-7 mol /dm3 Kw = [H3O+]*[OH-] = 10-7*10-7 = 10-14 mol2/dm6 -log Kw = -log ([H3O+]*[OH-])= -log [H3O+]+ -log[OH]=-log 10-14 pKw = pH + pOH = 14 The effect of temperature on the water dissociation constant Temperature (◦C) 0 15 25 50 100 Kw pKw 0,11 . 10 -14 0,45 . 10 -14 14,96 14,35 . 10 .-14 vid-14 Kw= 1,01,0 10 25 C 14,00 13,26 12,29 H2O + H2O 5,5 . 10 -14 51 . 10 -14 H3O+ + OH- DH> 0 Weak acids HA + H2O K= [H3O+] . [A- ] [HA] . [H2O] H3O+ + AKa=K . [H2O] = [H3O+] . [A- ] [HA] Ka = acid dissociation constant Ka => acid strength => higher value => stronger acid. pKa = -log Ka => lower value => stronger acid Some acid dissociation constants Ka 104 pKa -4 103 -3 Hydrochloric acid Sulphuric acid HCl H2SO4 Nitric acid Oxalic acid Phosphoric acid HNO3 H2C2O4 H3PO4 1 0.06 0.007 -1 1.25 2.15 Salicylic acid Citric acid C7H6O3 C6H8O7 0.001 7.10-4 2.97 3.13 Ascorbic acid C6H4O2(OH) 1.10-4 4.17 4 Acetic (etanoic) acid HAc Carbonic acid CH3COOH 1.8.10-5 H2CO3 4.2.10-7 Ammonium ion NH4+ 4.75 6.37 5.5.10-10 9.26 Weak bases BH + H2O K= [BH2+] . [OH-] . [BH] [H2O] BH2+ + OHKb=K . [H2O] = [BH2+] . [OH-] [BH] Kb = base dissociation constant Kb => base strength => higher value => stronger base pKb = -logKb => lower value => stronger base Bases Kb pKb Litium hydroxide Sodium hydroxide Ammonia LiOH NaOH NH3 2.3 0.63 1.8.10-5 -0.36 0.2 4.74 Hydrogen carbonate ion Acetate ion Nitrate ion Chloride ion HCO3- 2.4.10-8 7.62 CH3COONO3Cl- 5.5.10-10 9.25 10-15 15 10-18 18 Kw connects Ka and Kb for a corresponding acid/ base pair, such as CH3COOH/CH3COOKa * Kb = Kw = 10-14 pKa + pKb = pKw = 14 (at 25 ºC) Calculate pKa of ethanoic acid, HAc (CH3COOH) We know that c= 0.01M We measure pH HAc+H2O Cstart Ceq 0.1 0.1- 10-pH H3O+ +Ac0 10-pH 0 10-pH Ka = [H3O+]*[Ac-] / [HAc] = 10-pH* 10-pH /0.1- 10-pH= pKa= -log Ka = Calculate pH in 0,1 M ethanoic acid, HAc H3O+ +Ac- HAc Cstart Ceq 0.1 0.1-X 0 X 0 X pKa = 4.75 (see CDB) Ka = [H3O+]*[Ac-] / [HAc] = 10-4.75 = X2/0.1-X ~ X2/0.1 if x is small X2 = 0.1* 10-4.75 X = (0.1* 10-4.75 )½ pH = -log [X] = -log[(0.1* 10-4.75 )½] = 2.88 18.2 Buffer solutions • In pure water pH= 7. • Addition of small amounts of acid or base gives big changes in pH • That can be great problem, especially in biological systems. But there are ways to make a solution that can be quite pH stable. • A buffer resist changes in pH when a strong acid or base is added A Buffer: a mixture of a weak acid and its corresponding base The equilibrium: HA(aq) +H2O H3O+(aq) + A-(aq) If strong acid is added (fully dissociated, contains mainly H3O+) => reaction goes to the left (Le Chatelier’s principle) => little change in pH (-log [H3O+]) A Buffer: a mixture of a weak acid and its corresponding base The equilibrium: HA(aq) +H2O H3O+(aq) + A-(aq) • If a strong base is added => OH- reacts with H3O+ to form water => • equilibrium goes to the right => Restore the [H3O+] => little change in pH How to prepare a buffer: • Mix a weak acid and its corresponding (conjugate) base e.g. CH3COOH and CH3COO-Na+. • Mix a weak base and its corresponding (conjugate) e.g. NH3 and NH4Cl. • Add strong base to an excess of weak acid. • Add strong acid to an excess of weak base. Add strong base to an excess of weak acid (HA) The reaction of HA and water: HA(aq) +H2O H3O+(aq) + A-(aq) The reaction of HA and strong base (NaOH): HA + NaOH → H2O + Na+A- When you add some NaOH to a HAsolution your mixture will consist of all the above particles, but in particular the weak acid and it’s corresponding base HA/A- Add strong acid to an excess of weak base (BH) The reaction of BH and water: BH + H2O BH2+ + OH- The reaction of B and strong acid (HCl): HCl + BH → BH2+Cl- When you add some HCl to a BH-solution your mixture will consist of all the above particles, but in particular the weak base and it’s corresponding acid BH/BH2+ The Hydrogen ion concentration and pH of a buffer can be calculated from the expression for the acid dissociation constant: [H3O+] = Ka* [HA] /[A-] take –log on both sides -log [H3O+] = -log Ka+ -log [HA] /[A-] identify pH = pKa -log([HA] /[A-]) You have to be able to derive the equation yourself! Exercises 1. Which combination will form a buffer solution? A. 100 cm3 of 0.10 mol dm–3 hydrochloric acid with 50 cm3 of 0.10 mol dm–3 sodium hydroxide. B. 100 cm3 of 0.10 mol dm–3 ethanoic acid with 50 cm3 of 0.10 mol dm–3 sodium hydroxide. C. 50 cm3 of 0.10 mol dm–3 hydrochloric acid with 100 cm3 of 0.10 mol dm–3 sodium hydroxide. D. 50 cm3 of 0.10 mol dm–3 ethanoic acid with 100 cm3 of 0.10 mol dm–3 sodium hydroxide. 2. Buffer solutions resist small changes in pH. A phosphate buffer can be made by dissolving NaH2PO4 and Na2HPO4 in water, in which NaH2PO4 produces the acidic ion and Na2HPO4 produces the conjugate base ion. (i) Deduce the acid and conjugate base ions that make up the phosphate buffer and state the ionic equation that represents the phosphate buffer. (ii) Describe how the phosphate buffer minimizes the effect of the addition of a strong base, OH– (aq), to the buffer. Illustrate your answer with an ionic equation. (iii) Describe how the phosphate buffer minimizes the effect of the addition of a strong acid, H+(aq), to the buffer. Illustrate your answer with an ionic equation. Answers 1. Which combination will form a buffer solution? B. 100 cm3 of 0.10 mol dm–3 ethanoic acid with 50 cm3 of 0.10 mol dm–3 sodium hydroxide. You have both ethanoic acid and sodium ethanoate 2. (i) Acid: H2PO4–; (Conjugate) base: HPO42–; H2PO4–(aq) H+(aq) + HPO42–(aq); (ii) strong base/OH– replaced by weak base (H2PO42–, and effect minimized) / strong base reacts with acid of buffer / equilibrium in (i) shifts in forward direction; OH–(aq) + H2PO4–(aq) → H2O(l) + HPO42–(aq); (iii) strong acid/H+ replaced by weak acid (H2PO4–, and effect minimized) / strong acid reacts with base of buffer / equilibrium in (i) shifts in reverse direction; H+(aq) + HPO42–(aq) → H2PO4–(aq); 18.3 Salt hydrolysis • The positive and negative ion in a salt can be neutral or act as an acid or a base • Cations (positive ions) can act as acids and anions (negative ions) can act as bases The acetate (ethanoate) ion HAc + H2O H3O+ + Ac- acid Ac- + H2O base pKa(HAc)= 4.75 base HAc + OH- pKb(Ac-)= 9.25 acid The ethanoate ion is salt of a WEAK acid (ethanoic acid) and thus basic More basic ions • • • • CNHCO3CO32PO43- Salts of weak acids The chloride ion HCl + H2O H3O+ + Cl- acid Cl- + H2O base pKa(HCl)= - 4 base HCl + OH- pKb(Cl-)= 18 acid The chloride ion is salt of a STRONG acid (hydrochloric acid) and thus so week so it is neutral (>pKw) More ions with no acid/base character • • • • Na+ K+ Ca2+ NO3- SO42ClO4Cl- • Derives from strong acids and bases => no acid-base activity The ammonium ion NH3 + H2O NH4+ + OH- base acid NH4+ + H2O acid H3O+ + NH3 pKb(NH3)= 4.74 pKa(NH4+)= base Ammonium ion is salt of a WEAK base (ammonia) and thus acidic Metallic ions with high charge • Metallic ions with high charge, e.g. Al3+ and Fe3+, form complexes with water: • Al(H2O)63+ and Fe(H2O)63+. The electronegative effect of the ion weakens the O-H bond in water molecules: [Fe(H2O)6]3+(aq) + H2O [Fe(OH)(H2O)5]3+(aq)+ H3O+(aq) An acidic solution 18.4 Acid-base titrations • Strong acid – Strong base • Weak acid – Strong base • Strong acid – Weak base Strong acid – Strong base HCl + NaOH NaCl + H2O Start with 10 ml 0.1 M HCl (pH=1). Titrate with 0.1M NaOH • When 90% of the base been added: HCl ~0.01 => pH = 2 • When 99% of the base been added: HCl ~0.001 => pH = 3 • When 101% of the base been added: [OH-] = 0.001 pH =11 Weak acid – strong base CH3COOH + NaOH CH3COONa + H2O • When strong base is added the pH gradually increase. • At equivalence point all acid is consumed, pH increase rapidly. • The salt of the weak acid is a weak base => pH > 7 at equivalence point. • At ½ equivalence point [HA] =[A-] => pH => pKa Titration simulations at http://chem-ilp.net/labTechniques/AcidBaseIdicatorSimulation.htm 18.5 Indicators • A weak acid/base where the colours of the protonated and ionized forms are different HIn H+ + InRed Blue The colour depends both on pH and the pKavalue. => Different indicators change their colours at different pH Structures of BTB at different pH pH 7.3 Increasing [OH-]/pH Indicators change colour around their pKa-values How to choose indicator? • If you titrate CH3COOH with NaOH the pH will be above 7 at the equivalence point => choose an indicator that change colour above 7 e.g. phenolphthalein (pKa =9.6), range 8.3 – 10.0. Rapid pH changes in that area. - If you titrate NH3 with HCl the pH will be under 7 at the equivalence point => choose an indicator that change colour under 7 e.g. methyl orange(pKa = 3.7), range 3.1 – 4.4. Rapid pH changes in that area.