Chapter 6
advertisement

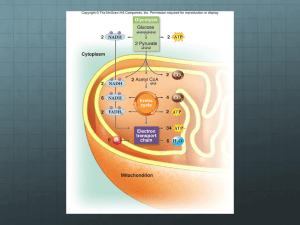
Chapter 6 Cellular Respiration: Obtaining Energy from Food Laua Coronado Chap 6 Bio 10 Biology and : Marathoners versuSocietys Sprinters – Sprinters do not usually compete at short and long distances. – Natural differences in the muscles of these athletes favor sprinting or longdistance running. Laua Coronado Chap 6 Bio 10 Muscle Fibers –The muscles that move our legs contain two main types of muscle fibers: • Slow twitch fibers –Generate less power –Last longer –Generate ATP using oxygen • Fast twitch fibers –Generate more power –Fatigue much more quickly –Can generate ATP without using oxygen Laua Coronado Chap 6 Bio 10 ENERGY FLOW AND CHEMICAL CYCLING IN THE BIOSPHERE – Animals depend on plants to convert solar energy to: • Chemical energy of sugars • Other molecules we consume as food – Photosynthesis: • Uses light energy from the sun to power a chemical process that makes organic molecules. Laua Coronado Chap 6 Bio 10 Producers and Consumers – Plants and other autotrophs (self-feeders): • Make their own organic matter from inorganic nutrients. – Heterotrophs (other-feeders): • Include humans and other animals that cannot make organic molecules from inorganic ones. – Autotrophs are producers because ecosystems depend upon them for food. – Heterotrophs are consumers because they eat plants or other animals. Laua Coronado Chap 6 Bio 10 Laua Coronado Chap 6 Bio 10 Figure 6.1 Chemical Cycling between Photosynthesis and Cellular Respiration – The ingredients for photosynthesis are carbon dioxide and water. • CO2 is obtained from the air by a plant’s leaves. • H2O is obtained from the damp soil by a plant’s roots. – Chloroplasts in the cells of leaves: • Use light energy to rearrange the atoms of CO2 and H2O, which produces – Sugars (such as glucose) – Other organic molecules – Oxygen Laua Coronado Chap 6 Bio 10 Cellular Respiration – Plant and animal cells perform cellular respiration, a chemical process that: • Primarily occurs in mitochondria • Harvests energy stored in organic molecules • Uses oxygen • Generates ATP – The waste products of cellular respiration are: • CO2 and H2O • Used in photosynthesis Laua Coronado Chap 6 Bio 10 Cellular Respiration – Animals perform only cellular respiration. – Plants perform: • Photosynthesis and • Cellular respiration Laua Coronado Chap 6 Bio 10 Sunlight energy enters ecosystem C6H12O6 Photosynthesis CO2 Glucose Carbon dioxide O2 Oxygen H2O Water Cellular respiration ATP drives cellular work Heat energy exits ecosystem Laua Coronado Chap 6 Bio 10 Figure 6.2 CELLULAR RESPIRATION: AEROBIC HARVEST OF FOOD ENERGY – Cellular respiration is: • The main way that chemical energy is harvested from food and converted to ATP • An aerobic process—it requires oxygen Laua Coronado Chap 6 Bio 10 CELLULAR RESPIRATION: AEROBIC HARVEST OF FOOD ENERGY –Cellular respiration and breathing are closely related. • Cellular respiration requires a cell to exchange gases with its surroundings. –Cells take in oxygen gas. –Cells release waste carbon dioxide gas. • Breathing exchanges these same gases between the blood and outside air. Laua Coronado Chap 6 Bio 10 CO2 O2 Breathing Lungs CO2 O2 Cellular respiration Laua Coronado Chap 6 Bio 10 Muscle cells Figure 6.3 Oxidation Glucose loses electrons (and hydrogens) C6H12O6 Glucose 6 O2 6 Oxygen CO2 6 Carbon dioxide Reduction Oxygen gains electrons (and hydrogens) Laua Coronado Chap 6 Bio 10 Figure 6.UN02 H2O Water The Role of Oxygen in Cellular Respiration – Cellular respiration can produce up to 38 ATP molecules for each glucose molecule consumed. – During cellular respiration, hydrogen and its bonding electrons change partners. • Hydrogen and its electrons go from sugar to oxygen, forming water. • This hydrogen transfer is why oxygen is so vital to cellular respiration. Laua Coronado Chap 6 Bio 10 Redox Reactions – Chemical reactions that transfer electrons from one substance to another are called: • Oxidation-reduction reactions or • Redox reactions for short – The loss of electrons during a redox reaction is called oxidation. – The acceptance of electrons during a redox reaction is called reduction. – During cellular respiration glucose is oxidized while oxygen is reduced. Laua Coronado Chap 6 Bio 10 Glycolysis Citric Acid Cycle ATP ATP Laua Coronado Chap 6 Electron Transport ATP Bio 10 Figure 6.UN03 Why does electron transfer to oxygen release energy? • When electrons move from glucose to oxygen, it is as though the electrons were falling. • This “fall” of electrons releases energy during cellular respiration. • Cellular respiration is: –A controlled fall of electrons –A stepwise cascade much like going down a staircase Laua Coronado Chap 6 Bio 10 1 O2 2 H2 Release of heat energy H2O Laua Coronado Chap 6 Bio 10 Figure 6.4 NADH and Electron Transport Chains – The path that electrons take on their way down from glucose to oxygen involves many steps. – The first step is an electron acceptor called NAD+. • The transfer of electrons from organic fuel to NAD+ reduces it to NADH. – The rest of the path consists of an electron transport chain, which: • Involves a series of redox reactions • Ultimately leads to the production of large amounts of ATP Laua Coronado Chap 6 Bio 10 e e Electrons from food e e NADH NAD 2 H Stepwise release of energy used to make ATP 2 e 2 e 1 2 2 H Hydrogen, electrons, and oxygen combine to produce water Laua Coronado Chap 6 O2 H2O Bio 10 Figure 6.5 An Overview of Cellular Respiration – Cellular respiration: • Is an example of a metabolic pathway, which is a series of chemical reactions in cells – All of the reactions involved in cellular respiration can be grouped into three main stages: • Glycolysis • The citric acid cycle • Electron transport Laua Coronado Chap 6 Bio 10 Mitochondrion Cytoplasm Cytoplasm Animal cell Plant cell Cytoplasm Mitochondrion High-energy electrons carried by NADH Glycolysis Glucose High-energy electrons carried mainly by NADH Citric Acid Cycle 2 Pyruvic acid ATP Electron Transport ATP ATP Laua Coronado Chap 6 Bio 10 Figure 6.6 The Three Stages of Cellular Respiration – Stage 1: Glycolysis • A six-carbon glucose molecule is split in half to form two molecules of pyruvic acid. • These two molecules then donate high energy electrons to NAD+, forming NADH. • Three steps – Uses two ATP molecules per glucose to split the sixcarbon glucose – Makes four additional ATP directly when enzymes transfer phosphate groups from fuel molecules to ADP • Produces a net of two molecules of ATP per glucose molecule. Laua Coronado Chap 6 Bio 10 INPUT OUTPUT 2 ATP 2 ADP Glucose Key Carbon atom Phosphate group High-energy electron Energy investment phase Laua Coronado Chap 6 Bio 10 Figure 6.7-1 INPUT OUTPUT NADH NAD 2 ATP 2 ADP Glucose Key Carbon atom Phosphate group High-energy electron NAD NADH Energy investment phase Laua Coronado Energy harvest phase Chap 6 Bio 10 Figure 6.7-2 INPUT OUTPUT NADH NAD 2 ATP 2 ADP 2 ATP 2 ADP 2 Pyruvic acid Glucose Key Carbon atom Phosphate group High-energy electron 2 ADP NAD 2 ATP NADH Energy investment phase Laua Coronado Energy harvest phase Chap 6 Bio 10 Figure 6.7-3 Enzyme P ADP ATP P P Laua Coronado Chap 6 Bio 10 Figure 6.8 Stage 2: The Citric Acid Cycle – Completes the breakdown of sugar. – In the citric acid cycle, pyruvic acid from glycolysis is first “prepped.” – The citric acid cycle: • Extracts the energy of sugar by breaking the acetic acid molecules all the way down to CO2 • Uses some of this energy to make ATP • Forms NADH and FADH2 Laua Coronado Chap 6 Bio 10 INPUT OUTPUT Oxidation of the fuel generates NADH (from glycolysis) NAD (to citric acid cycle) NADH CoA Pyruvic acid Pyruvic acid loses a carbon as CO2 Acetic acid CO2 Laua Coronado Coenzyme A Chap 6 Bio 10 Acetic acid attaches to coenzyme A Figure 6.9 Acetyl CoA INPUT OUTPUT Citric acid Acetic acid 2 CO2 ADP P ATP Citric Acid Cycle 3 NAD 3 NADH FAD FADH2 Acceptor molecule Laua Coronado Chap 6 Bio 10 Figure 6.10 Stage 3: Electron Transport – Releases the energy your cells need to make the most of their ATP. – The molecules of the electron transport chain are built into the inner membranes of mitochondria. • The chain functions as a chemical machine that uses energy released by the “fall” of electrons to pump hydrogen ions across the inner mitochondrial membrane. • These ions store potential energy. Laua Coronado Chap 6 Bio 10 Stage 3: Electron Transport – When the hydrogen ions flow back through the membrane, they release energy. • The hydrogen ions flow through ATP synthase. • ATP synthase: –Takes the energy from this flow –Synthesizes ATP Laua Coronado Chap 6 Bio 10 Space between membranes H H Electron carrier Protein complex Inner mitochondrial membrane Electron flow Matrix H H FADH2 FAD H H H H H H NAD H H H 1 2 NADH H H H O2 2 H H2O ADP H H H ATP synthase Electron transport chain Laua Coronado ATP P Chap 6 Bio 10 Figure 6.11 ETC Inhibitor – Cyanide is a deadly poison that: • Binds to one of the protein complexes in the electron transport chain • Prevents the passage of electrons to oxygen • Stops the production of ATP Laua Coronado Chap 6 Bio 10 The Versatility of Cellular Respiration – In addition to glucose, cellular respiration can “burn”: • Diverse types of carbohydrates • Fats • Proteins Laua Coronado Chap 6 Bio 10 Food Polysaccharides Fats Proteins Sugars Fatty acids Amino acids Glycerol Glycolysis Citric Acid Cycle Acetyl CoA Electron Transport ATP Laua Coronado Chap 6 Bio 10 Figure 6.12 Cytoplasm Mitochondrion NADH 2 2 Glycolysis 2 Pyruvic acid NADH 6 NADH 2 FADH2 2 Acetyl CoA Citric Acid Cycle Electron Transport 2 ATP 2 ATP About 34 ATP by direct synthesis by direct synthesis Glucose Laua Coronado Chap 6 Maximum per glucose: by ATP synthase Bio 10 Figure 6.13 About 38 ATP FERMENTATION: ANAEROBIC HARVEST OF FOOD ENERGY – Some of your cells can actually work for short periods without oxygen. – Fermentation is the anaerobic (without oxygen) harvest of food energy. – After functioning anaerobically for about 15 seconds: • Muscle cells will begin to generate ATP by the process of fermentation – Fermentation relies on glycolysis to produce ATP. Laua Coronado Chap 6 Bio 10 Laua Coronado Chap 6 Bio 10 FERMENTATION: ANAEROBIC HARVEST OF FOOD ENERGY – Glycolysis: • Does not require oxygen • Produces two ATP molecules for each glucose broken down to pyruvic acid – Pyruvic acid, produced by glycolysis, is • Reduced by NADH, producing NAD+, which keeps glycolysis going. – In human muscle cells, lactic acid is a byproduct. Laua Coronado Chap 6 Bio 10 INPUT 2 ADP 2 P OUTPUT 2 ATP Glycolysis 2 NAD 2 NADH Glucose Laua Coronado Chap 6 Bio 10 2 NADH 2 NAD 2 Pyruvic 2 H acid Figure 6.14 2 Lactic acid The Process of Science: Does Lactic Acid Buildup Cause Muscle Burn? – Observation: Muscles produce lactic acid under anaerobic conditions. – Question: Does the buildup of lactic acid cause muscle fatigue? – Hypothesis: The buildup of lactic acid would cause muscle activity to stop. – Experiment: Tested frog muscles under conditions when lactic acid could and could not diffuse away. Laua Coronado Chap 6 Bio 10 Battery Battery Force measured Force measured Frog muscle stimulated by electric current Solution allows diffusion of lactic acid; muscle can work for twice as long Solution prevents diffusion of lactic acid Laua Coronado Chap 6 Bio 10 Figure 6.15 The Process of Science: Does Lactic Acid Buildup Cause Muscle Burn? – Results: When lactic acid could diffuse away, performance improved greatly. – Conclusion: Lactic acid accumulation is the primary cause of failure in muscle tissue. – However, recent evidence suggests that the role of lactic acid in muscle function remains unclear. Laua Coronado Chap 6 Bio 10 Fermentation in Microorganisms – Fermentation alone is able to sustain many types of microorganisms. – The lactic acid produced by microbes using fermentation is used to produce: • Cheese, sour cream, and yogurt dairy products • Soy sauce, pickles, olives • Sausage meat products Laua Coronado Chap 6 Bio 10 Fermentation in Microorganisms – Yeast are a type of microscopic fungus that: • Use a different type of fermentation • Produce CO2 and ethyl alcohol instead of lactic acid – This type of fermentation, called alcoholic fermentation, is used to produce: • Beer • Wine • Breads Laua Coronado Chap 6 Bio 10 INPUT 2 ADP 2 P OUTPUT 2 ATP 2 CO2 released Glycolysis 2 NAD Glucose 2 NADH 2 NADH 2 NAD 2 Pyruvic 2 H acid Laua Coronado Chap 6 Bio 10 2 Ethyl alcohol Figure 6.16a Evolution Connection: Life before and after Oxygen – Glycolysis could be used by ancient bacteria to make ATP when little oxygen was available, and before organelles evolved. – Today, glycolysis: • Occurs in almost all organisms • Is a metabolic heirloom of the first stage in the breakdown of organic molecules Laua Coronado Chap 6 Bio 10 Billions of years ago 2.1 2.2 2.7 O2 present in Earth’s atmosphere 0 First eukaryotic organisms Atmospheric oxygen reaches 10% of modern levels Atmospheric oxygen first appears 3.5 Oldest prokaryotic fossils 4.5 Origin of Earth Laua Coronado Chap 6 Bio 10 Figure 6.17 Heat C6H12O6 Sunlight O2 ATP Cellular respiration Photosynthesis H2O CO2 Laua Coronado Chap 6 Bio 10 Figure 6.UN06 C6H12O6 6 O2 6 Laua Coronado Chap 6 CO2 Bio 10 6 H2 O Approx. 38 Figure 6.UN07 ATP Oxidation Glucose loses electrons (and hydrogens) CO2 C6H12O6 Electrons (and hydrogens) ATP H2O O2 Reduction Oxygen gains electrons (and hydrogens) Laua Coronado Chap 6 Bio 10 Figure 6.UN08 Mitochondrion O2 NADH 2 2 NADH Glycolysis NADH 2 FADH2 2 Acetyl CoA 2 Pyruvic acid Glucose 6 Citric Acid Cycle 4 CO2 2 CO2 2 ATP by direct synthesis Electron Transport by direct synthesis 2 ATP H2O About 34 ATP by ATP synthase About 38 ATP Laua Coronado Chap 6 Bio 10 Figure 6.UN09