Chapter 3 part I
advertisement

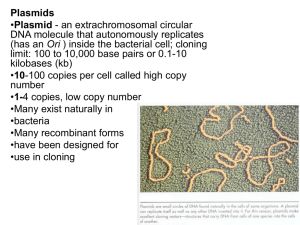
Recombinant DNA Technology Recombinant DNA • Protocols that transfer genetic information (DNA) from one organism to another. • Gene cloning links eukaryotic genes to small bacterial or phage DNAs and inserting these recombinant molecules into bacterial hosts. • One can then produce large quantities of these genes in pure form. Recombinant DNA - Gene Cloning DNA from a source organism is cleaved with restriction endonuclease and inserted into a cloning vector. The recombinant vector is introduced into a host cell. Recombinant cells are identified and grown. Five Basic Steps in Gene Cloning • The first is to choose the appropriate DNA to be cloned, genomic or cDNA. • Produce a collection of DNA fragments of size suitable for inserting into appropriate vectors. • Insert DNA fragments into the vector using DNA ligase (DNA ligation.) • Introduce DNA fragments into a population of bacteria (transformation.) • Select the colonies containing desired sequence from the “library.” Restriction Enzymes Restriction enzymes (restriction endonucleases) cut double-stranded DNA into smaller pieces. Bacteria use these as defense against DNA from bacteriophage. DNA is cut between the 3′ hydroxyl group of one nucleotide and the 5′ phosphate group of the next - restriction digestion. Restriction Enzymes • Restriction enzymes do not cut bacteria’s own DNA because the recognition sequences are modified. • Methylases add methyl groups after replication; makes sequence unrecognizable by restriction enzyme. Restriction Enzymes • Bacterial restriction enzymes can be isolated from cells. • DNA from any organism will be cut wherever the recognition site occurs. • EcoRI (from E. coli) cuts DNA at this sequence: GAATTC Restriction Enzymes The sequence is palindromic—it reads the same in both directions from the 5′ end. EcoRI occurs about once every four genes in prokaryotes. DNA can be chopped into small pieces containing a few genes. Restriction Enzymes Restriction endonucleases are named using the 1st three letters of their name from the Latin name of their source microorganism Hind III – First letter is from the genus H from Haemophilus – Next two letters are the 1st two letters of the species name in from influenzae – Sometimes the strain designation is included “d” from strain Rd – If microorganism produces only 1 restriction enzyme, end the name with Roman numeral I Hind I – If more than one restriction enzyme is produced, the others are numbered sequentially II, III, IV, etc. Restriction Enzymes • These enzymes can recognize 4-bp, 6-bp, 8-bp sequences • The frequency of cuts lessens when the recognition sequence is longer • A 6-bp cutter will yield DNA fragments averaging ~ 4000-bp or 4 kilobases (4kb) in length Restriction Enzymes • Many restriction endonucleases make staggered cuts in the 2 DNA strands – This leaves single-stranded overhangs, called sticky ends that can base-pair together briefly – This makes joining 2 different DNA molecules together much easier • Staggered cuts occur when the recognition sequence usually displays twofold symmetry, palindromes Staggered Cleavage Blunt-end Cleavage Restriction Enzymes • Heteroschizomers (isochizomers) recognize the same DNA sequence but are from different organisms. • Restriction nuclease that recognize the same DNA sequence but use a different cutting site – they are also called neoschizomers. • Restriction nuclease that produce the same nucleotide extension are called isocaudomers. Restriction Enzymes • These enzymes cut DNA strands reproducibly in the same place, which is extremely useful in gene analysis • Isoschizomers cleave a sequence only if the cytosines of the recognition site are not methylated whereas another will cut the same sequence if these cytosines are methylated. • For example, HpaII cuts only nonmethylated CCGG sites, and MspI cuts this sequence regardless of cytosine methylation. Neoschizomers Annealing of complementary extensions Gel Electrophoresis • After DNA is cut, fragments of different sizes can be separated by gel electrophoresis. • Mixture of fragments is place on a well in a porous gel. An electric field is applied across the gel. Negatively charged DNA fragments move towards positive end. • Smaller fragments move faster than larger ones. Restriction sites mapping Restriction sites mapping Plasmid Cloning Vectors Plasmids are small, circular DNA molecules that are maintained as independent extrachromosomal entities. F plasmids carry information for their own transfer from one cell to another. R plasmids encode resistant to antibiotics. Cryptic plasmids have no apparent functional coding genes. Plasmids can easily incorporate foreign DNA. Plasmids are readily taken up by bacterial cells. Plasmids then act as vectors, DNA carriers that move genes from one cell to another. Each plasmid has a sequence that functions as an origin of DNA replication. Vectors to Carry DNA Sequences A vector should have four characteristics: • Ability to replicate independently of the host cell • A recognition sequence for a restriction enzyme (cloning site) • One or more selectable/reporter genes • Small size in comparison with host’s chromosomes Vectors to Carry DNA Sequences Plasmids have all these characteristics. • Plasmids are small, many have only one restriction site. • Genes for antibiotic resistance can be used as reporter genes. • And they have an origin of replication and can replicate independently. Plasmid Cloning vector pBR322 • pBR322 illustrates cloning methods simply – Resistance for 2 antibiotics • Tetracycline • Ampicillin – Origin of replication between the 2 resistance genes – Only 1 site for several restriction enzymes Cloning using pBR322 Clone a foreign DNA into the PstI site of pBR322 Cut the vector to generate the sticky ends Cut foreign DNA with PstI also – compatible ends Combine vector and foreign DNA with DNA ligase to seal sticky ends Now transform the plasmid into E. coli 4-37 Cloning using pBR322 If new DNA is inserted at that PstI restriction site, it inactivates the gene for ampicillin resistance. Plasmid then has gene for tetracyclin resistance, but not for ampicillin. This can be used to select for host cells with new DNA. Cloning using pBR322 The cleaved plasmid DNA preparation is treated with enzyme alkaline phosphatase to remove the 5’ phosphate groups from the linearized plasmid DNA. Transformation and selection Traditional method involves incubating bacterial cells in concentrated calcium salt solution The solution makes the cell membrane leaky, permeable to the plasmid DNA – competent cells. Newer method uses high voltage to drive the DNA into the cells in process called electroporation 4-40 Transformation and selection A natural transformation process often entails The binding of double-stranded DNA to component of the cell wall. Entry of the DNA into an inner periplasm. Transmission of one strand into the cytoplasm while the other one is degraded. If the DNA is linear molecule, integration into host chromosome. If the introduced DNA is a plasmid, it is maintained in the cytoplasm after the second strand is synthesized. www.ncbi.nlm.nih.gov/bookshelf/picrender.fcgi... Transformation and selection Transformation produces bacteria with: Religated plasmid Religated insert Recombinants plasmid Identify the recombinants using the antibiotic resistance Grow cells with tetracycline so only cells with plasmid grow, not foreign DNA only (religated insert) Next, grow copies of the original colonies with ampicillin which kills cells with plasmid including foreign DNA (Figure 3.11) Clone a foreign DNA into the BamHI site Cells contain no plasmid are sensitive to both Amp and Tet. Cells contains intact plasmids are resistant to both. Cells contains inserted plasmids are resistant to Tet but sensitive to Amp. Screening with replica plating Replica plating transfers clone copies from original tetracycline plate to a plate containing ampicillin A sterile velvet transfer tool can be used to transfer copies of the original colonies Desired colonies are those that do NOT grow on the new ampicillin plate pUC and β - galactosidase Newer pUC plasmids have: Ampicillin resistance gene Multiple cloning site inserted into the gene lacZ’ coding for the enzyme β-galactosidase Clones with foreign DNA in the MCS disrupt the ability of the cells to make β-galactosidase Plate on media with a β-galactosidase indicator (X-gal) and clones with intact β-galactosidase enzyme will produce blue colonies Colorless (desirable) colonies should contain the plasmid with foreign DNA pUC and β - galactosidase Directional cloning Cut a plasmid with 2 restriction enzymes from the MCS Clone in a piece of foreign DNA with 1 sticky end recognizing each enzyme The insert DNA is placed into the vector in only 1 orientation Vector religation is also prevented as the two restriction sites are incompatible 4-49 • Digest plasmid using EcoRI and XhoI • Ligate cDNA into digested plasmids • Transformation – introduce recombinant plasmids into bacterial host cells. • Select transformants using blue-white screening. Summary First generation plasmid cloning vectors include pBR322 and the pUC plasmids pBR322 has 2 antibiotic resistance genes Variety of unique restriction sites for inserting foreign DNA Most of these sites interrupt antibiotic resistance, making screening straightforward pUC has Ampicillin resistance gene MCS that interrupts a β-galactosidase gene MCS facilitates directional cloning into 2 different restriction sites 4-52 Some antibiotics commonly used as selective agents Vector backbone exchange: SfiIx-SfiIy • Other bacteria, such as Bacillus subtilis and Agrobacterium tumefaciens, often act as the final host. • Cloning vectors that function in E. coli maybe provided with a second origin of replication. • In addition, a number of plasmid vectors have been constructed with a single broad-host-range origin of DNA replication so they can be used with a variety of microorganisms. Vector backbone exchange: SfiIx-SfiIy • The size of vector increases because of the additional sequence resulting in decreasing the amount of DNA that can be inserted. • Shuttle vectors are not efficiently propagated in the host cell. • Broad-host-range cloning vectors can be unstable and can be lost from a preferred host cells. • The chimeric vectors are engineered. Vector backbone exchange: SfiIx-SfiIy Vector backbone exchange: SfiIx-SfiIy • Cell without any plasmid and those with plasmids without chloramphenicol resistance gene cannot grow in the presence of chloramphenicol. • Plasmids that do not carry the origin of replication or that contain E. coli origin of replication will not be replicated in the host cell. • Only cells that carry the chimeric plasmid with the origin of replication in the host cell and chlorampenicol resistance gene will be selected. Making a genomic library • The first is to choose the appropriate DNA to be cloned, genomic or cDNA. • Produce a collection of DNA fragments of size suitable for inserting into appropriate vectors – partial digestion. • Insert DNA fragments into the vector using DNA ligase (DNA ligation.) • Introduce DNA fragments into a population of bacteria (transformation.) • Select the colonies containing desired sequence from the “library.” Making a genomic library Making a genomic library Vectors to Carry DNA Sequences Plasmids can be used for genes of 10,000 bp or less. Most eukaryote genes are larger than this. Viruses can be used as vectors—e.g., bacteriophage. The genes that cause host cell to lyse can be cut out and replaced with other DNA. Vectors to Carry DNA Sequences Bacterial plasmids don’t work for yeasts because the origins of replication use different sequences. A yeast artificial chromosome (YAC) has been created: contains yeast origin of replication, plus yeast centromere and telomere sequences. Also contains artificial restriction sites and reporter genes How many clones do we need? • The sum of the inserted DNA in the clones of the library should be three or more times the amount of the DNA in the genome. • For example, if a genome has 4 x 106 bp and the average size of an insert is 1,000 bp, then 12,000 clones are required for threefold coverage. • For human genome (3.3 x 109 bp), about 80,000 BAC clones that have an average insert size of 150,000 bp compose a library with fourfold coverage.