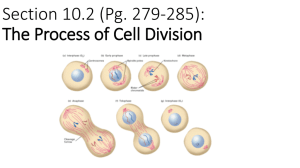
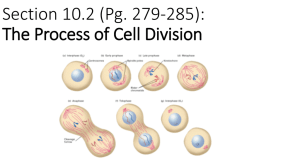
Chapter 29
Epigenetic
Effects Are
Inherited
29.1 Introduction
• Epigenetic effects can result from modification of a
nucleic acid after it has been synthesized or by the
perpetuation of protein structures.
Figure 29.01: Replication of a methylated
site produces hemimethylated DNA, in
which only the parental strand is
methylated.
Figure 29.02: Heterochromatin is
created by proteins that associate with
histones.
29.1 Introduction
• prion – A proteinaceous infectious agent that
behaves as an inheritable trait, although it
contains no nucleic acid.
– Examples are PrPSc, the agent of scrapie in sheep
and bovine spongiform encephalopathy, and PSI,
which confers an inherited state in yeast.
29.2 Heterochromatin Propagates from a
Nucleation Event
• Heterochromatin is nucleated at a specific sequence
and the inactive structure propagates along the
chromatin fiber.
• Genes within regions of heterochromatin are
inactivated.
• The length of the inactive region varies from cell to cell;
as a result, inactivation of genes in this vicinity causes
position effect variegation (PEV).
29.2 Heterochromatin Propagates from a
Nucleation Event
Figure 29.04: Extension of heterochromatin inactivates genes.
29.2 Heterochromatin Propagates from a
Nucleation Event
• Similar spreading effects occur at telomeres (telomeric
silencing) and at the silent cassettes in yeast mating
type.
29.3 Heterochromatin Depends on
Interactions with Histones
• HP1 is the key protein in forming mammalian
heterochromatin, and acts by binding to methylated
Structure from Protein Data Bank 1KNE. S. A. Jacobs
histone H3.
and S. Khorasanizadeh, Science 295 (2002): 20802083.
Photo reproduced from G. Lomberk, L. Wallrath, and R. Urrutia,
Genome Biol. 7 (2006): p. 228. Used with permission of Raul A.
Urrutia and Gwen Lamberk, Mayo Clinic.
Figure 29.06: HP1 contains a chromodomain and a chromoshadow domain. Methylation of
histone H3 creates a binding site for HP1.
29.3 Heterochromatin Depends on
Interactions with Histones
Figure 29.07: Binding of HP1 to methylated histone H3 forms a trigger for silencing.
29.3 Heterochromatin Depends on
Interactions with Histones
• Rap1 initiates formation of heterochromatin in yeast by
binding to specific target sequences in DNA.
• The targets of Rap1 include telomeric repeats and
silencers at HML and HMR.
• Rap1 recruits Sir3 and Sir4, which interact with the Nterminal tails of H3 and H4.
• Sir2 deacetylates the N-terminal tails of H3 and H4 and
promotes spreading of Sir3 and Sir4.
29.3 Heterochromatin Depends on
Interactions with Histones
Figure 29.08: Formation of
heterochromatin is initiated when Rap1
binds to DNA.
29.3 Heterochromatin Depends on
Interactions with Histones
• RNAi pathways promote heterochromatin formation at
centromeres.
29.4 Polycomb and Trithorax Are
Antagonistic Repressors and Activators
• Polycomb group proteins (Pc-G) perpetuate a state of
repression through cell divisions.
Figure 29.09: Pc-G proteins do not initiate repression, but are
responsible for maintaining it.
29.4 Polycomb and Trithorax Are
Antagonistic Repressors and Activators
• The PRE is a DNA sequence that is required for the
action of Pc-G.
• The PRE provides a nucleation center from which Pc-G
proteins propagate an inactive structure.
• Trithorax group proteins (trxG) antagonize the actions of
the Pc-G.
• Pc-G and trxG can bind to the same PRE with opposing
effects.
29.5 X Chromosomes Undergo Global
Changes
• dosage compensation – Mechanisms employed to
compensate for the discrepancy between the presence
of two X chromosomes in one sex but only one X
chromosome in the other sex.
Figure 29.10: Different means of
dosage compensation are used to
equalize X chromosome expression in
male and female.
Figure 29.11: X-linked variegation is caused
by the random inactivation of one X
chromosome in each precursor cell.
29.5 X Chromosomes Undergo Global
Changes
• constitutive heterochromatin – The inert state of
permanently nonexpressed sequences, such as satellite
DNA.
• facultative heterochromatin – The inert state of
sequences that also exist in active copies; for example,
one mammalian X chromosome in females.
29.5 X Chromosomes Undergo Global
Changes
• One of the two X chromosomes is inactivated at random
in each cell during embryogenesis of eutherian
mammals.
• single X hypothesis – The theory that describes the
inactivation of one X chromosome in female mammals.
• In exceptional cases where there are >2 X
chromosomes, all but one are inactivated (the n–1 rule).
29.5 X Chromosomes Undergo Global
Changes
• The Xic (X inactivation
center) is a cis-acting region
on the X chromosome that is
necessary and sufficient to
ensure that only one X
chromosome remains alive.
• Xic includes the Xist gene,
which codes for an RNA that
is found only on inactive X
chromosomes.
Figure 29.12: X-inactivation involves stabilization of
Xist RNA, which coats the inactive chromosome.
http://www.youtube.com/watch?v=Y9vXhmI5FXM
RNAi
• http://www.nature.com/nrg/multimedia/rnai/
animation/index.html
Crispr
http://www.youtube.com/watch?v=9IgLrOEsauk
29.5 X Chromosomes Undergo Global
Changes
• Xist recruits Polycomb complexes, which modify
histones on the inactive X.
• The mechanism that is responsible for preventing Xist
RNA from accumulating on the active chromosome is
unknown.
Figure 29.13: Xist RNA produced from the Xic locus accumulates on the future inactive X (Xi).
Adapted from A. Wutz and J. Gribnau, Curr.
Opin. Genet. Dev. 17 (2007): 387-393.
29.6 Chromosome Condensation Is Caused
by Condensins
• SMC (structural maintenance
of chromosome) proteins are
ATPases that include
condensins and cohesins.
• A heterodimer of SMC proteins
associates with other subunits.
Figure 29.15: (A) The basic architecture of condensin and
cohesin complexes. (B) Condensin and cohesin consist of Vshaped dimers of two SMC proteins interacting through
their hinge domains.
Adapted from T. Hirano, Nat. Rev. Mol. Cell Biol. 7 (2006): 311-322.
29.6 Chromosome Condensation Is Caused
by Condensins
• Condensins cause chromatin
to be more tightly coiled by
introducing positive supercoils
into DNA.
• Condensins are responsible
for condensing chromosomes
at mitosis.
• Chromosome-specific
condensins are responsible
for condensing inactive X
chromosomes in C. elegans.
Figure 29.18: Condensins may form a
compact structure by bending at the
hinge, causing DNA to become
compacted.
29.7 CpG Islands Are Subject to
Methylation
• Most methyl groups in DNA are found on cytosine on
both strands of the CpG doublet.
• Replication converts a fully methylated site to a
hemimethylated site.
• DNA methyltransferase – An enzyme that adds a
methyl group to a specific target sequence in DNA.
29.7 CpG Islands Are Subject to
Methylation
Figure 29.20: The state of methylated sites could be
perpetuated by an enzyme (Dnmt1) that recognizes
only hemimethylated sites as substrates.
29.7 CpG Islands Are Subject to
Methylation
• demethylase – An enzyme that removes a methyl
group, typically from DNA, RNA, or protein.
• de novo methyltransferase – An enzyme that adds a
methyl group to an unmethylated target sequence on
DNA.
• Hemimethylated sites are converted to fully methylated
sites by a maintenance methyltransferase.
• TET proteins convert 5-methylcytosine to 5hydroxymethylcytosine to lead to DNA demethylation.
29.7 CpG Islands Are Subject to
Methylation
Figure 29.21: The state of methylation is controlled by three types of enzyme.
29.8 DNA Methylation Is Responsible for
Imprinting
• Paternal and maternal alleles may
have different patterns of
methylation at fertilization.
• Methylation is usually associated
with inactivation of the gene.
• When genes are differentially
imprinted, survival of the embryo
may require that the functional
allele is provided by the parent with
the unmethylated allele.
Figure 29.23: The typical pattern
for imprinting is that a methylated
locus is inactive.
29.8 DNA Methylation Is Responsible for
Imprinting
• Survival of heterozygotes for imprinted genes is different,
depending on the direction of the cross.
• Imprinted genes occur in clusters and may depend on a
local control site where de novo methylation occurs
unless specifically prevented.
29.9 Oppositely Imprinted Genes Can Be
Controlled by a Single Center
• Imprinted genes are controlled by methylation of cisacting sites.
• Methylation may be responsible for either inactivating or
activating a gene.
Figure 29.24: The ICR is methylated on
the paternal allele, where Igf2 is
active and H19 is inactive.
29.10 Epigenetic Effects Can Be Inherited
• Epigenetic effects can result from modification of a
nucleic acid after it has been synthesized or by the
perpetuation of protein structures.
• Epigenetic effects may be inherited through generations
(transgenerational epigenetics).
29.10 Epigenetic Effects Can Be Inherited
Figure 29.27: Acetylated histones are conserved and distributed at random to the daughter
chromatin fibers at replication.
29.11 Yeast Prions Show Unusual
Inheritance
• The Sup35 protein in its
wild-type soluble form is a
termination factor for
translation.
• Sup35 can also exist in an
alternative form of
oligomeric aggregates, in
which it is not active in
protein synthesis.
Figure 29.28: The state of the Sup35
protein determines whether
termination of translation occurs.
29.11 Yeast Prions Show Unusual
Inheritance
• The presence of the
oligomeric form causes
newly synthesized protein
to acquire the inactive
structure.
Figure 29.29: Newly synthesized Sup35 protein is
converted into the [PSI+] state by the presence of
preexisting [PSI+] protein.
29.11 Yeast Prions Show Unusual
Inheritance
• amyloid fibers – Insoluble fibrous protein polymers with
a cross β-sheet structure, generated by prions or other
dysfunctional protein aggregations (such as in
Alzheimer’s).
• Conversion between the two forms is influenced by
chaperones.
• The wild-type form has the recessive genetic state psi–
and the mutant form has the dominant genetic state
PSI+.
29.12 Prions Cause Diseases in Mammals
• kuru – A human neurological disease caused by prions.
• The protein responsible for scrapie exists in two forms:
the wild-type noninfectious form PrPC, which is
susceptible to proteases, and the disease-causing PrPSc,
which is resistant to proteases.
29.12 Prions Cause Diseases in Mammals
• The neurological disease can be transmitted to mice by
injecting the purified PrPSc protein into mice.
• The recipient mouse must have a copy of the PrP gene
coding for the mouse protein.
Figure 29.31: A PrpSc protein can only
infect an animal that has the same
type of endogenous PrPC protein.
29.12 Prions Cause Diseases in Mammals
• The PrPSc protein can perpetuate itself by causing the
newly synthesized PrP protein to take up the PrPSc form
instead of the PrPC form.
• Multiple strains of PrPSc may have different
conformations of the protein.