ppt
advertisement

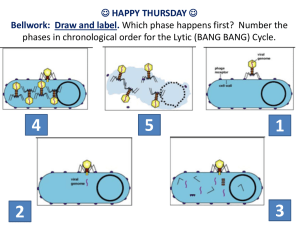
Applications of the RG Study of molecular biology of RNA viruses Pathogenesis Vaccine development Vectors Other applications…. VPM 700 May’06 Molecular Biology of RNA viruses Provide insights into the many aspects that control the virus life cycle - the location and boundaries of the cis-acting elements - the secondary structure of promotor regions - the functional domains of the trans-acting proteins - the gene stop-start signals - AND MANY MORE…… Pathogenesis Genetic determinant of virulence by H5N1 virus in mice (Hatta et al., Science, 2001) A/HongKong/483/97 (HK483) – Highly Pathogenic A/HongKong/486/97 (HK486) – Mildly Pathogenic Prepared the single-gene reassortant viruses In vivo characterization PB2 Protein is responsible for the difference in virulence Amino acid residue at position 627 influence the outcome of infection H7N2 influenza in the U.S. H7N2 LPAI virus circulation in NY and NJ LBM’s since 1994 LBM H7N2 virus served as source of infection for commercial poultry operation - 1997-1998, Pennsylvania - 2001-2002, Pennsylvania - 2002, Virginia, West Virginia, North Carolina - 2003, Connecticut - 2004, Delaware, Maryland LBM H7N2 virus may serve as source of infection for human - 2003, 1st human case of H7N2 infection in New York Determinant of pathogenicity Not totally defined An optimal constellation of viral genes is required for pathogenicity expression Hemagglutinin (HA) structure is a primary determinant of pathogenicity in chickens and turkeys “HA0 cleavage into HA1 and HA2” - Necessary for fusion peptide to be exposed - Essential for virus to become infectious All AI viruses : trypsin-like enzyme (respiratory, intestinal) HPAI viruses : ubiquitous protease (systemic) Key features for HA cleavability The amino acid sequence at the cleavage site - multiple basic a.a. The carbohydrate in the vicinity of the cleavage site - no glycosylation at Asn11 Receptor binding site Asn11 Cleavage site Molecular features of H7N2 virus 1994-1995, 1999 Cleavage site sequence (HA1/HA2)* P-E-N-P-K-T-R/G 1995-1999, 2002 P-E-N-P-K-P-R/G 23 1995, 1998 P-E-K-P-K-T-R/G 2 1998-2005 P-E-K-P-K-P-R/G 426 2002, 2004-2005 P-E-K-P-K-K-R/G 82 Year observed No. of isolates * Basic amino acids (Lysine(K) and Arginine(R)) are in bold character. Is H7N2 virus progress toward HPAI virus ? What additional changes are required ? 6 Pathogenic Potential of H7N2 Virus Virus Parent Mutant Mutant Mutant Mutant Mutant Mutant Mutant Mutant Mutant Mutant Mutant Mutant sequence In vitro In vivo replication plaquing in ECE efficiency PEKP---KKR/G 1 PEKR---KKR/G 2 PEKK---KKR/G---- inefficient 3 PEKP--RKKR/G 4 PEKP--KKKR/G---- inefficient 5 PEKR--RKKR/G 6 PEKR--KKKR/G---- inefficient 7 PEKP-KKKKR/G 8 PEIP-KKKKR/G 9 PEKPKKRKKR/G 10 PEIPKKRKKR/G 11 PENPKKRKKR/G 12 PETPKKRKKR/G Positive control (CK/Chile/02 (H7N3)) mortality virulence - 0/8 - - 0/8 - - 0/8 - + 0/8 ++ - 0/8 - ++ 2/8 +++ - 0/8 - + 0/8 + + 0/8 + +++ 3/8 ++++ +++ 4/8 ++++ +++ 5/8 ++++ +++ 5/8 ++++ +++ 1/8 +++ Transcription Plasmids (8) Rescue of viruses carrying mutated HA genes P Avian – PB2 T P Avian – PB1 T P Avian – PA T P Mutated H7 T P Avian – NP T P Avian – N1 T P Avian – M T P Avian – NS T Expression Plasmids (4) + P PB2 A P PB1 A P PA A P NP A Co-transfection 293T Amplification 10-day eggs Reassortant H7N1 strains with different H7 gene Reverse genetics using helper virus 4 Expression Plasmids P PB2 A P PB1 A P PA A P NP A + Transcription Plasmids P H7 HA T Co-transfection Selection of HP reassortant by plaque assay in CEF 293T 24hr incubation HP reassortant 293T Helper virus infection (wild type H7 virus) 48hr incubation no HP reassortant 4 Expression Plasmids P PB2 A P PB1 A P PA A P NP A + Transcription Plasmids P H7 HA T Co-transfection M 3 PEKP--RKKR/G M 5 PEKR--RKKR/G M 7 PEKP-KKKKR/G M 8 PEIP-KKKKR/G M 9 PEKPKKRKKR/G M 10 PEIPKKRKKR/G M 11 PENPKKRKKR/G M 12 PETPKKRKKR/G CK/Chile/02-H7 Egret/HK/02-H5 CK/Indonesia/04-H5 √ √ √ √ Selection of HP reassortant by plaque assay in CEF 293T 24hr incubation 293T Helper virus infection (wild type H7 virus) Chile/02, Egret/02, Indonesia/04, H7-M3, M5, M9, M12 reassortants 48hr incubation All other H7 mutants Pathogenicity of reassortants rescued by helper virus rescue system ECE Virus CEF Chickens Cleavage site sequence EID50/ml MDT 0.3ug trypisn no trypsin Mortality MDT Virus titer h-M-3 PEKP--RKKR 7.9 <2 4X10^6 (1mm) 2X10^6 (>2mm) 8/8 2.9+2.0 6.4+0.1(2) h-M-5 PEKR--RKKR 9.5 <2 5X10^7 (1mm) 3X10^6 (>2mm) 8/8 1.9+0.8 - h-M-9 PEKPKKRKKR 9.2 <2 3X10^7 (1-2mm) 8X10^6 (>2mm) 8/8 2.9+1.2 6.3+1.3 (2) h-M-12 PETPKKRKKR 8.5 <2 2X10^7 (1-2mm) 1X10^7 (>2mm) 8/8 3.6+0.7 5.6+0.5(4) PEKPKTCSPLSRCRETR 8.2 <2 8/8 3.6+1.3 5.8+0.8(4) h-M-chile 2X10^8 (2mm) 2X10^7 (>2mm) MDT: Mean death time (average days taken to kill the embryo or chickens b Number of birds that died / number of birds inoculated. c The virus titer is express as the log 10 EID50 per milliliter ± standard deviation. d No birds survived a Vaccine Development Non-structural (NS1) protein, a novel target for the development of influenza vaccines Functions of the NS1 protein - Inhibit the early production of cellular antiviral mRNA - display host unique PL (PDZ domain ligand) motifs at the C-terminus for PDZ domains involved in cell signaling pathways (Science, 2006) Genomes RNA segments (bp) Protein (aa) 1. Polymerase (basic) 2 (2341) PB2 (759) 2. Polymerase (basic) 1 (2341) PB1 (757), *PB1-F2 3. Polymerase (acidic) (2233) PA (716) 4. Hemagglutinin (1775) HA (565) 5. Nucleoprotein (1565) NP (498) 6. Neuraminidase (1413) NA (454) 7. Matrix (1027) M1 (252) Virion constituents M2 (97) 8. Nonstructural (890) NS2(NEP)(121) NS1 (230) Infected cells A/turkey/Oregon/71 (H7N3) Low virulence AI isolate from a turkey flock with mild respiratory illness Genetically distinct stocks exist TK/OR/71-SEPRL (Suarez et al., 1999) - Low passage stock of the field isolates - Encode a full length NS1 protein (230aa) TK/OR/71-del (Norton et al., 1987) - Unknown passage history - 10 nt deletion in the NS gene resulting in a truncated NS1 protein TK/OR/71-D-dels (Lee et al., 2005) - Derived from TK/OR/71-del stock by passaging in old ECE - Different size of deletion in the middle of the NS gene stop * SEPRL NS genes * del 880 bp 705 bp 644 bp 487 bp 890bp 880bp 380-389 (10nt) * D-del 1 705bp 267-451 (185nt) * D-del 2 644bp 218-463 (246nt) * D-del 3 204-606 (403nt) 487bp translation RNA binding SEPRL Effector domain 1 230 73 230 aa 117 ▼ del NS1 124 aa 80 ▼ D-del 1 64 D-del 2 ▼ 90 aa 160 ▼ 148 aa 59 D-del 3 ▼ 144 aa Transcription Plasmids (8) Rescue of viruses carrying different NS genes P A/WSN/33 – PB2 T P A/WSN/33 – PB1 T P A/WSN/33 - PA T P Avian – H5 T P A/WSN/33 - NP T P Avian – N1 T P A/WSN/33 - M T P NS T SEPRL (890bp) del (880bp) D-del 1 (705bp) D-del 2 (689bp) D-del 3 (644bp) D-del 4 (487bp) Expression Plasmids (4) + P PB2 A P PB1 A P PA A P NP A Co-transfection 293T Amplification 10-day eggs Reassortant H5N1 strains with different NS gene DIVA strategy (Differentiating Infected from Vaccinated Animals) Use of heterologous strain containing the same hemagglutinin subtype as the field virus but a different neuraminidase in an oil-emulsion vaccine (Beard, 1986; Stone, 1987) H7N3 subtype vaccine was used to control H7N1 LPAI virus in Italy (2000-2002) H7N3 subtype vaccine is being used to control H7N2 LPAI virus in Connecticut Limitations 1) availability of heterologous vaccine (The H7N3 outbreak in Northern Italy in 2002.) 2) degree of homology between the hemagglutinin gene in vaccine and challenge virus Reverse genetics for rapid generation of influenza vaccine banks WSN-PB2 WSN-PB1 WSN-PA P P P T WSN-NP P T T WSN-M P WSN-PB1 WSN-PA WSN-NP T WSN-NS P T WSN-PB2 T 6 transcription plasmids from WSN/33 (H1N1) 4 expression plasmids + H5: A/CK/PA/13609/93 (H5N2) H7: A/TK/VA/55/02 (H7N2) H5 or H7 N1 or N8 P P T T N1: A/DK/Anyang/01 (H5N1) N8: A/DK/NY/191255-59/02 (H5N8) Transfection 293T 102.0-4.0 EID50 / ml 2 days ECE Amplification 2-3 days rH5N1, rH7N1 rH5N8, rH7N8 Reassortant vaccine strains 108.0 EID50 / ml Use of Reverse Genetics for Development of DIVA Vaccines (Lee et al., Vaccine 2004) Use of plasmid-based reverse genetic system to produce viruses with specific HA and NA subtypes Post-Vaccination Groups *HI titer (s.d.) Post-Challenge **NI test Number of positive birds ***Virus titer (s.d.) N1 N2 N8 3dpi 5dpi 3dpi 5dpi H5N2 6.5 (0.9) 0/10 10/10 0/10 4/10 0/10 0.4 (0.6) 0 rH5N1 6.7 (0.8) 10/10 0/10 0/10 4/10 0/10 0.6 (1.1) 0 10/10 9/10 4.5 (0.5) 3.4 (0.6) Control H7N2 6.8 (0.9) 0/10 10/10 0/10 0/10 0/10 0 0 rH7N8 6.8 (1.1) 0/10 0/10 10/10 0/10 0/10 0 0 10/10 10/10 4.9 (0.4) 3.9 (0.9) Control *HI titer is expressed as log2 reciprocal of the endpoint in twofold sera dilution **NI test were performed with NI, N2 and N8 antigen and the number of positive samples were indicated ***Virus titer is expressed as log10 50% egg infective doses per milliliter Differentiation by indirect IFA assay H5N2 infected H5N2 vaccinated rH5N1 vaccinated H7N2 infected H7N2 vaccinated rH7N8 vaccinated An alternative DIVA approach on the basis of antibodies to NS1 The NS1 protein is nonstructural Killed vaccines for influenza are primarily made with whole virion The NS1 DIVA approach works well with horses receiving purified vaccines (Ozaki et al., 2001). Vaccinated chickens produce low levels of antibody to the NS1 protein, but virus-infected chickens will produce higher levels of NS1 antibody (Tumpey et al., 2005). Application – DIVA vaccine RNA binding SEPRL 1 Effector domain 73 230 230 aa D-del 1 90 aa D-del 2 163 aa DIVA strategy - truncated NS1 recombinant protein produced in E. coli can be used as coating antigen in ELISA format - seroconversion will be observed in infected, but not in vaccinated birds Application - Vector Insert foreign sequences * 705bp D-del 1 D-del-GFP 267-451 CRS NS1 Expression of foreign gene - GFP - HIS tag - Foreign antigenic epitope GFP 338 aa Application – Live Vaccine For humans, - cold-adapted live vaccine - FluMistTM was approved for use in the U.S. Potential problems of the use of live influenza vaccines in poultry - bird-to-bird transmission - vaccine-induced respiratory disease - potential for recombination with newly introduced AIV Application – Live Vaccines Genetically engineered live attenuating virus - changes in the PB2 gene - exchanging promoter region of the NA gene - generating viruses that have truncated NS1 TK/OR/71-del virus - attenuation in pathogenicity - decreased ability to replicate in chickens - inefficient bird-to-bird transmission => In ovo vaccination with attenuated live influenza virus that induces strong immune response, but has no infectious virus present in the hatched chicks. Other Application of RG - Antigenic drift study A 194 154 188 158 123 126 160 181 B 218 Antigenic drift occurs away from the vaccine strain with avian influenza virus (Lee et al., J Virology, 2004) The genetic changes of the HA1 gene were concentrated in or near the predicted antigenic sites in the protein. We also found a mutation that created a glycosylation site near the antigenic site => Using a mapping approach, amino acid changes that are important for immunity will be identified Receptor binding site 136137 175 113 71 270 C 40 275 VPM 700 May’06