Genetic screens, sevenless revisited, pathways and paper techniques
advertisement

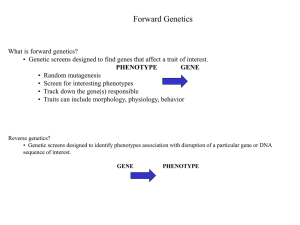
• • • • Genetic Screens Sevenless revisited Pathways you’ll want to know Quick review of techniques Genetic Screens Benefits of Drosophila • Only 4 chromosomes • Short generation time (10 days) • Lots of external features with visible mutations (bristles, wings, eyes, etc.) • A large number of human homologs • Ability to carry out large-scale genetic screens for mutations Forward Genetics 1. Create Random Mutations… EMS (ethyl methane sulphonate) introduces point mutations (Protocol by Lewis and Bacher 1968) 2. Screen for a phenotype of interest Be sure to design a simple screen that can be done in bulk 3. Clone gene from mutants of interest Involves lots of sequencing Making mutations • • • • EMS can be fed to flies… Typically causes point mutations Ave. mutation rate for a gene is 1:1000 Drawback is mosaicism (some cells carry mutation while others do not) • X-ray irradiation induces double-stranded DNA breaks that don’t cause mosaicism • Often large chromosomal rearrangements or deletions • About an order of magnitude less efficient than EMS Screening for phenotypes •Want a phenotype that is easy to identify, but specific enough to your question of interest •Need to minimize the background of mutants that don’t affect the process of interest •Sometimes a more laborious and specific screen saves you time in the end (less sequencing of irrelevant genes) Some obvious phenotypes one can screen for… Cloning the gene… FOR EMS-BASED MUTATIONS: •Single nucleotide polymorphisms (SNPs) are passed down from parents to offspring •A map of SNPs for flies exists •Mapping the inheritance of a phenotype to the inheritance of SNPs allows the rapid mapping of mutations to regions less than 50kb FOR X-RAY BASED MUTATIONS: •Because these are large scale chromosomal rearrangements or deletions, can often be detected cytologically in larval polytene chromosomes •Allows mutation to be mapped rapidly to a region and then IDed on Southern blots Sevenless revisited An omatidium is made up of 8 photoreceptors (R1-R8) & accessory cells The sevenless mutation is relatively easy to screen for… All 8 present Sevenless! Too many sevens Almost back to normal Suppressor & Enhancer Screens… Forward genetic screens can generate a variety of alleles of a gene • Amorphs (null mutations) • Weak hypomorphs (partial loss-of-function mutations) • Constitutively active (always on…no longer regulated) Supressor and enhancer screens can give one an idea about downstream effectors of the protein of interest Suppressor & Enhancer Screens con’t Constitutively active form of sevenless Causes a “rough” phenotype A screen for dominant suppressors of Sev receptor identified a loss-of-function Allele of drk Hypomorphic mutation in sevenless causes decrease in the number of R7 cells A screen for dominant suppressors that increase the number of R7 cells turned up a gain of function muant in sos And the final result… *GTP* GDP SH2 SH3 “sevenless in absentia” Well-used Pathways •GPCR-linked signaling •RTK-linked signaling •JAK-STAT pathway •Others… Model of G-proteinCoupled Activation CREB Receptor Tyrosine Kinases • Receptors dimerize in response to ligand binding • Cross-phosphorylation fully activates the receptors • They phosphorylate other residues – Recruit other proteins to these binding sites – These other proteins can then be activated by phosphorylation RTK Signaling Complex con’t… JAK/STAT: the TF is its own second messenger! Some signaling pathways are less conventional… Notch Delta Axon Guidance Signaling • Eph receptors are traditional RTKs • Semaphorins, netrins and slits signal through novel receptors What themes do you notice??? Quick review of techniques • • • • • Chimeras Transfection GFP as a marker of transfection Co-IP Yeast 2 hybrid Yeast two-hybrid What it tells you: Screen for interacting proteins How do you do it? Transfect yeast with designed plasmids: 1. Gene for protein A upstream of gene encoding GAL4 DNA binding domain, creating a fusion protein. 2. Genes from a library upstream of gene encoding GAL4 activation domain If the two proteins interact, the GAL4-AD will be brought into close proximity with the binding domain and will be able to initiate transcription of a reporter gene. Yeast two-hybrid What it looks like Positive control with colonies expressing beta-gal Negative control. Colonies do not express beta-gal Test protein. Amount of beta-gal production indicates strength of interaction Co-Immunoprecipitation (co-IP) G Why do we use it? To capture our protein of interest and look for protein-protein interactions 2. G How it works: 1. The Fc region of the antibody sticks to the bead. 2. Incubate the antibody beads with cell lysate to pull down the protein of interest (and anything else that stuck to it). 1. 3. G Co-Immunoprecipitation (co-IP) Question: Does Protein B Bind to Protein A?? Immunoprecipitate with Antibody to A and see if it Brings down protein B Protein B Antibodies stuck to the beads Protein A 3. G Protein A Use an antibody to A to confirm it’s there Protein B Use an antibody to B to see if it’s there… Antibodies for visualizing protein Note: This does not prove a direct interaction but it does suggest that the proteins interact in vivo. i.e. is it A-C-B?? What do you do if there are no antibodies specific to your protein? HA-tag Myc-tag Flag anti-HA antibody Protein of interest HA