Xeroderma Pigmentosum (XP)
advertisement

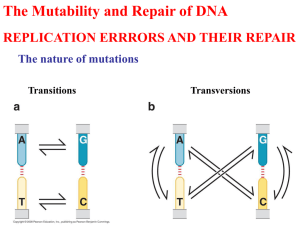
Repair of DNA and XP Errol C. Friedberg (2003) DNA damage and repair. Nature 421: 436-440. Xeroderma Pigmentosum (XP) Symptoms include: --- Extreme sensitivity to sunlight --- Early onset of skin cancer Why are XP patients sensitive to sunlight? 8 gens UV Healthy DNA NER Damaged DNA NO REPAIR Healthy DNA Xeroderma Pigmentosum (XP) Hereditary Cancer Syndromes involving defects in DNA Repair • Xeroderma pigmentosum..........Nucleotide Excision Repair • Hereditary Nonpolyposis......... Colon Cancer Mismatch DNA Repair • Ataxia Telangiectasia............... Double-Strand Break • Fanconi Anemia DNA ..............Crosslink Repair • Li-Fraumeni ..............................Nucleotide Excision Repair • Breast-Ovarian Cancer.............. Double Strand Breaks Nucleotide Excision Repair Daño y reparación del DNA Exogenous Metabolism Endogenous Cell Cycle Arrest DNA Damage DNA Repair DNA Replication Apoptosis Permanent Genetic Alteration Disease Types of DNA damage • Base Loss • Base modification & Deamination • Chemical Modification • Photodamage • Inter-strand crosslinks • DNA-protein crosslinks • Strand breakage Base loss Abasic site -loss of a nucleobase (apurinic or apyrimidinic) Deamination Potential Sites of modification/damage Chemical Damage Alkylation Oxidative damage UV-induced damage Pyrimidine dimers Note: Cytosine residues can also form dimers Types of Damage Repair • Photolyase • De-alkylation proteins (not catalytic) • Base Excision Repair • Nucleotide Excision Repair (GG and TC) • Recombination Repair • Error-prone Repair • Double strand Break Repair (if time permits) UV-responsive photolyases Direct reversal (de-alkylating proteins) Base Excision Repair Base Excision Repair BER NER Nucleotide Excision Repair (E.coli) Nucleotide Excision Repair (Global Genome Repair -Humans) Nucleotide Excision Repair (Transcription Coupled -Humans) NER Common features of GGR & TCR Mismatch Repair Recombination Repair Other possible mechanisms of recombination repair Mecanismos de Reparación del DNA (DSB) Recombinacion Homologa RAD50 MRE11 NSB1 Error Prone Bypass (E. coli) Experimental evidence for Error prone repair (E.coli) Revertant in His- genes (umuC mutated strain) UV-responsive activation of the umuC gene DNA repair polymerases DNA polymerase Eta (XP-V) - addition of two dA residues across pyrimidine dimers DNA polymerase Zeta - addition of random residues across pyrimidine dimers Non-Homologous End Joining (Double Strand Breaks) Model for activation of DNA damage repair Damage & Repair • Multiple forms of DNA damage occur • These are repaired constantly by several mechanisms • Failure to repair damage leads to mutations • Often defects in damage sensing machinery or DNA repair processes can be correlated with increased incidence of diseases such as cancer Factors involved in Damage Sensing Apoptosis and DNA Repair • Objectives: – 1) Understand that programmed cell death or apoptosis is genetically controlled and is an important factor in tumour growth – 2) Describe the relationship of abnormal DNA repair to genetic instability and cancer-prone syndromes – 3) Describe DNA-damage activated cell-cycle checkpoints and how they prevent mutation and abnormal cellular division The Importance of DNA-dsb Repair Van Gent et al, 2001 Molecular DNA-dsb Repair BRCA1/2 Van Gent et al, 2001 DNA Damage-Induced Protein Interactions Nbs1 Mre11 DAPI H2AX BP53 BRCA1 0 0.5 Merged Rad50, Rad51 3 6 12 Time after IR (hrs) Indicators or Biomarkers of DNA Repair ? BRCA1/BRCA2 • Tumour suppressor genes associated with high risk of breast cancer at young age with bilateral tumour risk (BRCA1 also ovarian and prostate/colon; BRCA2 also ovarian, male breast) • These mutations account for 80% of familial breast cancer; yet only 5-10% of breast cancers are familial, the others are sporadic • NOT associated with sporadic breast cancer (unlike p53) • Both proteins involved in the repair of DNA double strand breaks and predispose to aberrant DNA replication and lead to mutations and cancer ATM BRCA1/2 proteins may have a role in homologous DNAdsb repair due to ATM, rad50 and rad51 interactions and mutant BRCA1/2 proteins can not interact appropriately HNPPC and Mismatch Repair • Hereditary non-polyposis colorectal cancer (HNPPC) is a disorder of faulty mismatch repair and genetic instability • evolution of multiple tumours occurs much more rapidly at young age (<50 yrs) and accounts for 2-4% of all colonic cancers • Have decreased ability to repair replication errors (RER+ phenotype) due to a lack of the MLH1 or MSH2 genes which normally remove incorrect DNA base pairs • Leads to DNA microsatellite instability (MSI) detectable on gel electrophoresis; interestingly MSI+ colon cancers have a better prognosis following therapy Microsatellite Instability (tandom repeats of DNA) is a sign of MMR-deficiency and may be due to MSH2-MLH1 mutations (IHC) = Diagnostic Testing Chromosomal Control and Cancer: Telomeres • Cells are capable of only a limited series of divisions before they arrest or senesce and chromosomal fusions and cell death • Telomeres caps of chromosome ends and function to prevent DNA loss during DNA replication and provide a cellular clock for cell proliferation • They consist of a specific sequence TTAGGGG associated with proteins (TEP, hTERT) • As somatic cells normally age, telomeres reduce in length due to decreased function of telomerase, an enzyme which is used by germline cells to maintain telomere length = Cancer Prevention or Tumour Suppression • In human tumours, telomerase activity is abnormally high leading to abnormal control of cell growth and proliferation Normal and Abnormal Telomere Functioning In Normal or Cancer Cells Harrington, 2001 SUMMARY -Apoptosis is triggered by a number of external stressors including chemo- or radiotherapy and is controlled by the p53, myc and bax-bcl-2 proteins -Distinct morphologic features define apoptotic cells secondary to caspase activity -The relative level of apoptosis versus cell proliferation determines selection of those mutant cells which may proliferate to form a tumour -Defects in DNA repair of UV-damage, DNA replication errors or DNA-dsbs can lead to genetic instability and genetic mutation -These defects lead to cancer-prone syndromes such as xeroderma pigmentosum, HNPPC and BRCA1-associated tumours in which patients are sensitive to specific DNA-damaging agents, develop cancers at an early age and have chromosomal instability in tissues