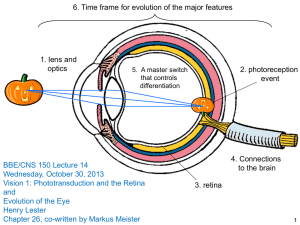
The G protein pathway in neuroscience
advertisement
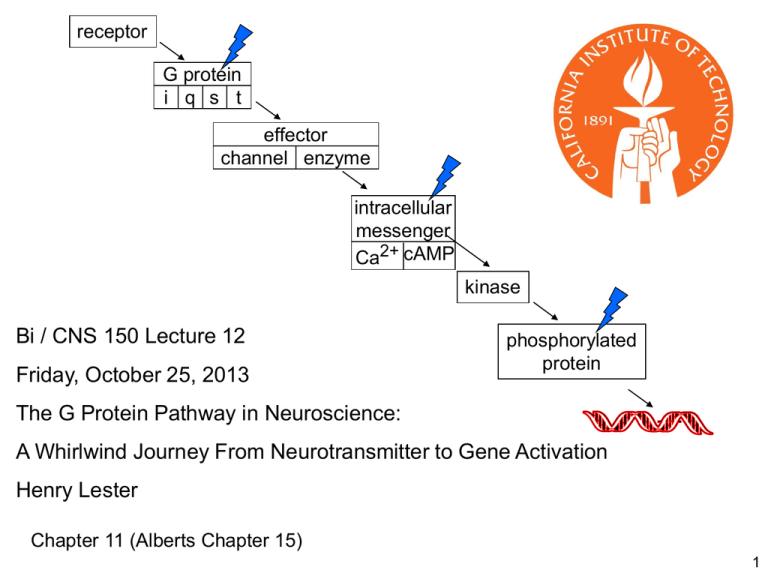
receptor G protein i q s t effector channel enzyme intracellular messenger Ca2+ cAMP kinase Bi / CNS 150 Lecture 12 Friday, October 25, 2013 phosphorylated protein The G Protein Pathway in Neuroscience: A Whirlwind Journey From Neurotransmitter to Gene Activation Henry Lester Chapter 11 (Alberts Chapter 15) 1 From previous lectures Proof of chemical synaptic transmission, 1921. Many details of the G protein pathway were first worked out for neuronal control of the heart Vagus nerve runs from the head to the heart Spontaneous heartbeats in both hearts are stopped by stimuli to the “upstream” vagus The diffusible substance: acetylcholine acting on muscarinic ACh receptors smoked drum 2 Some postsynaptic membranes contain G protein-coupled receptors (“metabotropic” receptors) rather than ligand-gated channels cytosol vesicles containing serotonin NH3+ HO N H G protein-coupled serotonin receptor vesicles containing acetylcholine O O N+(CH3)3 G protein-coupled (muscarinic) acetylcholine receptor vesicles containing dopamine HO HO H2 C C H2 NH3+ synaptic cleft G protein-coupled dopamine receptor cytosol 3 Several small-molecule transmitters serve as agonists for both ligand-gated channels & GPCRs (among vertebrates) Transmitter Ligand-gated channel GPCR ACh nicotinic AChR muscarinic AChR GABA GABAA GABAB glutamate iGluR mGluR serotonin 5-HT3 5-HTn, n = 1,2, 4-7 histamine (invertebrates only) Hn dopamine (invertebrates only) Dn 4 On a time scale of seconds (perhaps minutes), the language of the nervous system is still electricity; and we are still describing a set of mechanisms that manipulate impulse frequencies in individual neurons. 5 Plasma Membrane Components of the G Protein Pathway How fast? 100 ms to 10 s How far? Probably less 1 mm Neurotransmitter or hormone binds to receptor activates G protein Effector: enzyme or channel outside a Rasmussen et al., Nature 2011 PDB file 3SN6 GTP b g a GDP + Pi b g inside G protein-coupled receptors receptor G protein i q s t 1. All have 7 a-helices effector channel enzyme 2. There are about 1000 G protein-coupled receptors in the genome. intracellular messenger (Most are still “orphans”; their ligands are unknown) Ca2+ cAMP 3. Individual receptors respond to: (a) a low-molecular weight neurotransmitter such as serotonin, dopamine, or acetylcholine (b) a short protein (8-40 amino acids, a “peptide”) such as an endorphin (c) a relatively insoluble lipid such as anandamide, the endocannabinoid (d) an olfactory stimulus; or (e) light, in the eye (receptor = rhodopsin) 7 Structure of a heterotrimeric G protein: a molecular switch receptor β subunit G protein i q s t effector α subunit intracellular messenger GDP γ subunit PDF file: 1GOT Note the “propeller” in the b subunit which caps the a subunit, preventing either subunit from interacting with the effector (There is no effector in this structure): receptor Structure of a heterotrimeric G protein: a molecular switch G protein i q s t http://www.its.caltech.edu/~lester/Bi-150/G protein-alpha-beta-gamma.pdb Viewer required effector intracellular messenger Note the “propeller” in the b subunit which caps the a subunit, preventing either subunit from interacting with the effector (There is no effector in this structure): http://www.its.caltech.edu/~lester/Bi150/G protein-beta-only.pdb 9 From previous lectures How ”tight” is the gigaohm seal? acetylcholine in the pipette opens channels in the pipette 2. Chemically tight acetylcholine outside the pipette opens channels outside the pipette The seal compartmentalizes molecules. Molecules outside the pipette do not mix with molecules inside the pipette 10 receptor Gi protein effectors include some K+ channels G protein i q s t effector channel enzyme intracellular messenger Ca2+ cAMP no transmitter +Gbg Normally: released from Gi; Here: added by experimenter no additions n= 0 (closed) b g b g b g 3b. Mechanically tight Use weak suction. Excised “inside-out” patch allows access to the inside surface of the membrane no channel openings n = 1 (open) +Gbg n = 0 (closed) 11 receptor G protein-gated K channels inhibit neuronal (& cardiac) firing Resting GK EK -90 mV effector channel enzyme Voltage-gated Ligand-gated G protein-gated outside GEPSP EEPSP ~ -5 mV G protein i q s t GCl ECl -80 mV GNa GK GK ENa +50 mV EK -90 mV EK -90 mV intracellular messenger Ca2+ cAMP Capacitance cytosol = inside +60 additional K+ channels keep the membrane potential away from threshold, and therefore decrease firing rates mV -60 1 ms 5 G protein gated K+ channels (GIRKs) are inward rectifiers. When activated, they “latch” the cell quiet until excitatory stimuli finally succeed in depolarizing to threshold. E K GK + E EPSP GEPSP + E Cl GCl + E NaGNa DV = GK + G EPSP + G Cl + G Na Gi-coupled receptors usually inhibit neurons Gi directly activates some K channels Gi directly inhibits some voltage-gated Ca channels Gi directly inhibits adenylyl cyclase All these actions slow neuronal firing and decrease transmitter release 13 receptor Gq, Gs, and Gt protein effectors include some enzymes Gq G protein i q s t effector channel enzyme Enzyme Ca2+ in endoplasmic reticulum Ca2+ in cytosol intracellular messenger Ca2+ cAMP 14 phosphatidyl inositol 4,5 bisphosphate = PI(4,5)P2 Our first example of intracellular ligand-gated channels Alberts et al., Molecular Biology of the Cell, © Garland Science Figure on p.242 16 KCNQ channels PIP2 is necessary for keeping some K channels open. Gq activation leads to less PIP2 Result: some K channels close. These are called “M” channels, and are now termed the KCNQ family. because they were first discovered downstream from muscarinic receptors . . . A different muscarinic receptor subtype from the one that opens K channels in heart. Figure 11-11 17 receptor G protein i q s t Gq, Gs, and Gt protein effectors include some enzymes: effector channel enzyme Gs-coupled receptors often stimulate neurons & other cells intracellular messenger Ca2+ cAMP ATP 2+ Mg Mg2+ NH2 N N O O O -O P O P O P O CH2 O H H OOOH OH OH ATP cyclic AMP (cAMP) Gs NH2 N N N N N cyclase O O O P -O O N H H OH cyclic AMP (cAMP) See Figure 11-3 18 receptor G protein i q s t caffeine prolongs the intracellular messenger cAMP effector channel enzyme intracellular messenger Ca2+ cAMP ATP cyclic AMP (cAMP) NH2 N N Mg 2+ N O O O -O P O P O P O CH2 O H H OOOH OH OH NH2 N N N N cyclase O O O P -O O N H H OH Inhibited by caffeine phosphodiesterase AMP 19 receptor G protein i q s t cyclase cAMP ATP effector channel enzyme Inhibited by caffeine phosphodiesterase AMP intracellular messenger cAMP Ca2+ cGMP Phosphodiesterase inhibitors prolong the life of intracellular messengers cyclase cGMP GTP Inhibited by . . . phosphodiesterase GMP 20 intracellular messenger Ca2+ cAMP Intracellular messengers bind to proteins kinases A few ion channels (olfactory system, retina) phosphorylated protein NH2 N N Ca2+ and O O O P -O O N N H H OH cyclic AMP (cAMP) 21 Ca2+ or cAMP binds to kinase; this activates the kinase. intracellular messenger Ca2+ cAMP kinase phosphorylated protein Alberts 11-31 © Garland serine O N CHC O H CH2 OH Residue in target protein kinase phosphatase O N CHC O H CH2 O -O P O O 22 Example of ion channel phosphorylation: β-adrenergic receptors regulate accommodation in hippocampal neurons intracellular messenger Ca2+ cAMP kinase Apply norepinephrine phosphorylated protein Norepinephrine inhibits the SK (smallconductance, Ca2+ -activated K+) channel. Apply 8-bromo-cAMP Therefore the after-hyperpolarization (AHP) is smaller and spike trains are longer. Apply forskolin (then apply glutamate in the presence of TTX) epsp The norepinephrine effect is also mimicked by agents that mimic or increase cAMP. 1. phosphodiesterase.does not hydrolyze 8-bromo-cAMP 2. Forskolin activates cyclase Example of ion channel phosphorylation: β-adrenergic receptors regulate accommodation in hippocampal neurons intracellular messenger Ca2+ cAMP kinase Apply norepinephrine phosphorylated protein Norepinephrine inhibits the SK (smallconductance, Ca2+ -activated K+) channel. Apply 8-bromo-cAMP Apply forskolin (then apply glutamate in the presence of TTX) epsp Therefore the after-hyperpolarization (AHP) is smaller and spike trains are longer. The norepinephrine effect is also mimicked by agents that mimic or increase cAMP. 1. phosphodiesterase.does not hydrolyze 8-bromo-cAMP 2. Forskolin activates cyclase receptor Discussion G protein i q s t Selective advantage of such a complex pathway? The neurotransmitter or hormone does not directly influence the response--from the viewpoint of (a) Chemistry (b) Speed (c) Localization (to some extent) effector channel enzyme intracellular messenger Ca2+ cAMP All this amplification and indirect coupling requires energy! Limitations of the pathway: (a) Speed (b) co-operativity Further advantages / limitations? Suggestions in class: 25 receptor Genomic diversity of the G protein pathway ~ 1000 G protein-coupled receptors All have 7 helices G proteins all have 3 subunits There are ~ 18 a subunit genes in 4 major classes i, q, s, t ~ 5 b subunits ~ 3 g subunits G protein i q s t effector channel enzyme intracellular messenger Ca2+ cAMP and many “accessory proteins”. Now we discuss one There are 2 major types of effectors Channels affected by G proteins: ~5 known K channel genes ~4 Ca2+ channels Enzymes 3 major classes, each with 2 to 10 members 26 Regulators of G protein Signaling tune the kinetics of effector (GIRK channel) activation/deactivation CHO CHO Expressed: muscarinic ACh Receptor + GIRK . . . . . .+ RGS RGS4 GTP b g a a b g RGS GTP Gαi GDP + Pi 25 27 On a time scale of seconds (perhaps minutes), the language of the nervous system is still electricity; and we are still describing a set of mechanisms that manipulate impulse frequencies in individual neurons. Now we proceed to effects on a longer time scale (hours to days). Classical “Outside-in” Mechanisms for Long-term Actions on G Protein Pathways 28 Seymour Benzer’s early Drosophila learning mutants “Normal Drosophila learn to avoid an odorant associated with electric shock. A . . . mutant, dunce, has been isolated that fails to display this learning in spite of being able to sense the odorant and electric shock and showing essentially normal behavior in other respects.” Quinn, et al., PNAS 1974; Dudai et al., PNAS 1976 fluorescent lamp Two of Seymour Benzer’s early Drosophila learning mutants involve the cAMP system cyclase rutabaga cAMP ATP phosphodiesterase dunce AMP 30 intracellular messenger Ca2+ cAMP kinase phosphorylated protein kinase phosphorylated protein Nucleus 31 Many genes have a DNA sequence called “cAMP-Ca2+ responsive element” (CRE) intracellular messenger Ca2+ cAMP kinase Target or reporter gene CRE pCREB phosphorylated protein The transcription factor that binds to this CRE: “cAMP-Ca2+ responsive element binder” (CREB). O -O P O O Alberts et al., Molecular Biology of the Cell, © Garland Science from Lecture 12 outside receptor membrane b g G protein i q s t a b g a inside effector channel enzyme The pathway from GPCR to gene activation intracellular messenger Ca2+ cAMP cytosol kinase phosphorylated protein nucleus How fast? 10 s to days How far? Up to 1 m 33 A typical schematic drawing See also Figure 11-15 34 Henry Lester’s “office” hours continue all term Monday & Friday 1:15-2 PM Outside the Red Door End of Lecture 12 35 receptor G protein i q s t cyclase effector channel enzyme cAMP ATP Inhibited by caffeine phosphodiesterase AMP intracellular messenger cAMP Ca2+ cGMP Phosphodiesterase inhibitors prolong the life of intracellular messengers cyclase cGMP GTP Inhibited by Viagra, Cialis, Levitra phosphodiesterase GMP 36