Evolution 1/e
advertisement

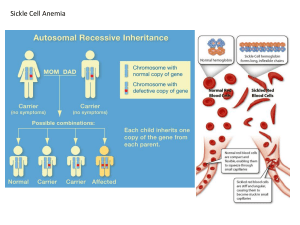
Read Chapter 7 of text We saw in chapter 6 that a cross between two individuals heterozygous for a dominant allele produces a 3:1 ratio of individuals expressing the dominant phenotype: to those expressing the recessive phenotype. For example brachydachtyly (shortening of the digits) displays this pattern of inheritance. In the early 1900’s when Mendel’s work was rediscovered there was confusion about how these simple patterns of inheritance affected populations. Why, for example, was not 3 of every 4 people a person with brachdactyly? Why did not dominant alleles replace recessive alleles? The confusion stemmed from confusing what was happening at the level of the individual with what occurs at the population level. Individual-level thinking enables us to figure out the result of particular crosses. Population level thinking however is needed to figure out how the genetic characteristics of populations change over time. It enables us to figure out quantitatively what is happening in a population as a result of evolution. Remember, evolution occurs when genotype frequencies change over time. Null models provide us with a baseline. They tell us what we expect to be the case if certain forces are not operating. The Hardy-Weinberg equilibrium tells us what we expect to happen to genotype frequencies when forces such as natural selection are not operating on a population. The Hardy-Weinberg model enables us to determine what allele and genotype frequencies we would expect to in a population if all that is happening is alleles are being randomly assigned to gametes and those gametes meet up at random. The Hardy-Weinberg model examines a situation in which there is one gene with two alleles A1 and A2. There are three possible genotypes A1A1, A2 A2,and A1 A2 Hardy and Weinberg used their model to predict what would happen to allele frequencies and genotype frequencies in the absence of any evolutionary forces. Their model produced three important conclusions The three conclusions of the H-W model. In the absence of evolutionary processes acting on them: 1. The frequencies of the alleles A1 and A2 do not change over time. 2. If we know the allele frequencies in a population we can predict the equilibrium genotype frequencies (frequencies of A1A1, A2 A2,and A1 A2). 3. A gene not initially at H-W equilibrium will reach H-W equilibrium in one generation. 1. No selection. › If individuals with certain genotypes survived better than others, allele frequencies would change from one generation to the next. 2. No mutation › If new alleles were produced by mutation or alleles mutated at different rates, allele frequencies would change from one generation to the next. 3. No migration › Movement of individuals in or out of a population would alter allele and genotype frequencies. 4. Large population size. › Population is large enough that chance plays no role. Eggs and sperm collide at same frequencies as the actual frequencies of p and q. › If assumption was violated and by chance some individuals contributed more alleles than others to next generation allele frequencies might change. This mechanism of allele frequency change is called Genetic Drift. 5. Individuals select mates at random. › Individuals do not prefer to mate with individuals of a certain genotype. If this assumption is violated allele frequencies will not change, but genotype frequencies might. Assume two alleles A1 and A2 with known frequencies (e.g. A1 = 0.6, A2 = 0.4.) Only two alleles in population so their allele frequencies add up to 1. Can predict frequencies of genotypes in next generation using allele frequencies. Possible genotypes are: A1A1 , A1A2 and A2A2 Assume alleles A1 and A2 enter eggs and sperm in proportion to their frequency in population (i.e. 0.6 and 0.4) Assume sperm and eggs meet at random (one big gene pool). Then we can calculate expected genotype frequencies. A1A1 : To produce an A1A1 individual, egg and sperm must each contain an A1 allele. This probability is 0.6 x 0.6 or 0.36 (probability sperm contains A1 times probability egg contains A1). Similarly, we can calculate frequency of A2A2. 0.4 x 04 = 0.16. Probability of A1A2 is given by probability sperm contains A1 (0.6) times probability egg contains A2 (0.4). 0.6 x 04 = 0.24. But, there’s a second way to produce an A1A2 individual (egg contains A1 and sperm contains A2). Same probability as before: 0.6 x 0.4= 0.24. Overall probability of A1A2 = 0.24 + 0.24 = 0.48. Genotypes in next generation: A1A1 = 0.36 A1A2 = 0.48 A2 A2= 0.16 Adds up to one. General formula for Hardy-Weinberg. Let p= frequency of allele A1 and q = frequency of allele A2. p2 + 2pq + q2 = 1. If there are three alleles with frequencies P1, P2 and P3 such that P1 + P2 + P3 = 1 Then genotype frequencies given by: P12 + P22 + P32 + 2P1P2 + 2P1 P3 + 2P2P3 Allele frequencies in a population will not change from one generation to the next just as a result of assortment of alleles and zygote formation. If the allele frequencies in a gene pool with two alleles are given by p and q, the genotype frequencies will be given by p2, 2pq, and q2. The frequencies of the different genotypes are a function of the frequencies of the underlying alleles. The closer the allele frequencies are to 0.5 the greater the frequency of heterozygotes. You need to be able to work with the Hardy-Weinberg equation. For example, if 9 of 100 individuals in a population suffer from a homozygous recessive disorder can you calculate the frequency of the disease causing allele? Can you calculate how many heterozygotes you would expect in the population? p2 + 2pq + q2 = 1. The terms in the equation represent the frequencies of individual genotypes. P and q are allele frequencies. It is vital that you understand this difference. 9 of 100 (frequency = 0.09) of individuals are homozygotes. What term in the H-W equation is that equal to? It’s q2. If q2 = 0.09, what’s q? Get square root of q2, which is 0.3. If q=0.3 then p=0.7. Now plug p and q into equation to calculate frequencies of other genotypes. p2 = (0.7)(0.7) = 0.49 2pq = 2 (0.3)(0.7) = 0.42 Number of heterozygotes = 0.42 times population size = (0.42)(100) = 42. There are three alleles in a population A1, A2 and A3 whose frequencies respectively are 0.2, 0.2 and 0.6 and there are 100 individuals in the population. How many A1A2 heterozygotes will there be in the population? Just use the formulae P1 + P2 + P3 = 1 and P12 + P22 + P32 + 2P1P2 + 2P1 P3 + 2P2P3 = 1 Then substitute in the appropriate values for the appropriate term 2P1P2 = 2(0.2)(0.2) = 0.08 or 8 people out of 100. Hardy Weinberg equilibrium principle identifies the forces that can cause evolution. If a population is not in H-W equilibrium then one or more of the five assumptions is being violated. If we relax the H-W assumption of no selection how does that affect allele frequencies? To quantify the strength of selection against a recessive allele we can use a parameter (s) called the selection coefficient to describe the reduction in fitness of one phenotype vs the other. For example pocket mice coat color is affected by a gene with two alleles D and d. D allele is dominant. DD: dark phenotype Dd: dark phenotype Dd: light phenotype On dark backgrounds light phenotype will be selected against. The higher the value of s the more strongly natural selection will act. The mouse coat color example is an example of frequency-independent selection. The fitness of a trait is not associated with how common the trait is. The commonest form of frequencyindependent selection is directional selection. Under directional selection one allele is consistently favored over the other allele so selection drives allele frequencies in only one direction towards a higher frequency of the favored allele. Eventually favored allele may replace other alleles and become fixed. Clavener and Clegg’s work on Drosophila. Two alleles for ADH (alcohol dehydrogenase breaks down ethanol) ADHF and ADHS Two Drosophila populations maintained: one fed food spiked with ethanol, control fed unspiked food. Populations maintained for multiple generations. Experimental population showed consistent long-term increase in frequency of ADHF Flies with ADHF allele have higher fitness when ethanol present. ADHF enzyme breaks down ethanol twice as fast as ADHS enzyme. Fig 5.13 Jaeken syndrome: patients severely disabled with skeletal deformities and inadequate liver function. Autosomal recessive condition caused by loss-of-function mutation of gene PMM2 codes for enzyme phosphomannomutase. Patients unable to join carbohydrates and proteins to make glycoproteins at a high enough rate. Glycoproteins involved in movement of substances across cell membranes. Many different loss-of-function mutations can cause Jaeken Syndrome. Team of researchers led by Jaak Jaeken investigated whether different mutations differed in their severity. Used HardyWeinberg equilibrium to do so. People with Jaeken syndrome are homozygous for the disease, but may be either homozygous or heterozygous for a given disease allele. Different disease alleles should be in Hardy-Weinberg equilibrium. Researchers studied 54 patients and identified most common mutation as R141H. Dividing population into R141H and “other” alleles. Allele frequencies are: Other: 0.6 and R141H: 0.4. If disease alleles are in H-W equilibrium then we would predict genotype frequencies of Other/other: 0.36 Other/R141H: 0.48 R141H/R141H: 0.16 Observed frequencies are: Other/Other: 0.2 Other/R141H: 0.8 R141H/R141H : 0 Clearly population not in H-W equilibrium. Researchers concluded that R141H is an especially severe mutation and homozygotes die before or just after birth. Thus, there is selection so H-W assumption is violated. Theory predicts that if an average individual carrying an allele has higher than average fitness that the frequency of that allele will increase from one generation to the next. Obviously, the converse should be true and a deleterious allele should decrease in frequency if its bearers have lower fitness. If the average fitness of an allele A when paired at random with other alleles in the population is higher than the average fitness of the population, then it will increase in frequency. Dawson (1970). Flour beetles. Two alleles at locus: + and l. +/+ and +/l phenotypically normal. l/l lethal. Dawson founded two populations with heterozygotes (frequency of + and l alleles thus 0.5). Expected + allele to increase in frequency and l allele to decline over time. Predicted and observed allele frequencies matched very closely. l allele declined rapidly at first, but rate of decline slowed. Fig 5.16a Dawson’s results show that when the recessive allele is common, evolution by natural selection is rapid, but slows as the recessive allele becomes rarer. Hardy-Weinberg explains why. When recessive allele (a) common e.g. 0.95 genotype frequencies are: AA (0.05)2 Aa (2 (0.05)(0.95) aa (0.95)2 0.0025AA 0.095Aa 0.9025aa With more than 90% of phenotypes being recessive, if aa is selected against expect rapid population change. When recessive allele (a) rare [e.g. 0.05] genotype frequencies are: AA (0.95)2 Aa 2(0.95)(0.05) aa (0.05)2 0.9025AA 0.095Aa 0.0025aa Fewer than 0.25% of phenotypes are aa recessive. Most a alleles are hidden from selection as heterozygotes. Expect only slow change in frequency of a. Dawson’s beetle work shows that deleterious rare alleles may be very hard to eliminate from a gene pool because they remain hidden from selection as heterozygotes. This only applies if the allele is not dominant. A dominant allele is expressed both as a heterozygote and a homozygote and so is always visible to selection. One way in which multiple alleles may be maintained in a population is through heterozygote advantage (also called overdominance). Classic example is sickle cell allele. Sickle cell anemia is a condition common among West Africans and those of West African descent. Under low oxygen conditions the red blood corpuscles are sickle shaped. Untreated the condition usually causes death in childhood. About 1% of West Africans have sickle cell anemia. A single mutation causes a valine amino acid to replace a glutamine in the alpha chain of hemoglobin The mutation causes hemoglobin molecules to stick together. Only individuals homozygous for the allele get sickle cell anemia. Individuals with only one copy of the allele (heterozygotes) get sickle cell trait (a mild form of the disease) Individuals with the sickle cell allele (one or two copies) don’t get malaria. Heterozygotes have higher survival than either homozygote (heterozygote advantage). Sickle cell homozygotes die of sickle cell anemia, many “normal” homozygotes die of malaria. Stabilizing selection thus favors sickle cell allele. A heterozygote advantage (or overdominance) results in a balanced polymorphism in a population. Both alleles are maintained in the population as the heterozygote is the best combination of alleles and a purely heterozygous population is not possible. Underdominance is when the heterozygote has lower fitness than either homozygote. This situation is In this case one or other allele will go to fixation, but which depends on the starting allele frequencies In some cases the costs and benefits of a trait depend on how common it is in a population. In this case the commoner a phenotype is the more successful it is. If two phenotypes are determined by single alleles one allele will go to fixation and the other be lost, but which one depends on the starting frequencies. In “flat” snails individuals mate face to face and physical constraints mean only individuals whose shells coil in the same direction can mate successfully. Higher frequencies of one coil direction leads to more mating for that phenotype and eventually it replaces the other types. Under negative frequency-dependent selection a trait is increasingly favored the rarer it becomes. Color polymorphism in Elderflower Orchid Two flower colors: yellow and purple. Offer no food reward to bees. Bees alternate visits to colors. How are two colors maintained in the population? Gigord et al. hypothesis: Bees tend to visit equal numbers of each flower color so rarer color will have advantage (will get more visits from pollinators). Experiment: provided five arrays of potted orchids with different frequencies of yellow orchids in each. Monitored orchids for fruit set and removal of pollinaria (pollen bearing structures) As predicted, reproductive success of yellow varied with frequency. 5.21 a Another example of negative frequencydependent selection involves a scaleeating cichlid fish in Lake Tanganyika. The fish come in left- and right-mouthed morphs. They attack their victims from behind. Because each morph always attacks the same side of its victims when the frequency of a morph increases the victims become good at guarding against attacks from that side. The common morph then suffers reduced feeding success and declines in abundance. As a result the morphs fluctuate in frequency over time. It is obvious that selection is a very powerful evolutionary force but how strong is mutation alone as an evolutionary force? To check: Two alleles A and a. Frequency of A = 0.9, a = 0.1. Assume A mutates to a at rate of 1 copy per 10,000 per generation (high rate, but within observed range) and all mutations occur in gametes. How much does this change gene pool in next generation? Hardy Weinberg genotypes in current generation: 0.81 AA, 0.18 Aa, 0.01 aa With no mutation allele frequency in gene pool 0.9 A, 0.1 a But mutation reduces frequency of A and increases frequency of a A a 0.9 - (0.0001)(0.9) 0.1 + (0.0001)(0.9) 0.89991A 0.10009a Not a big change. After 1000 generations frequency of A = 0.81. Mutation alone clearly not a powerful evolutionary force. But mutation AND selection make a very powerful evolutionary force. Lenski et al. studied mutation and selection together in an E. coli strain that did not exchange DNA (hence mutation only source of new variation). Bacteria grown in challenging environment (low salts and low glucose medium) so selection would be strong. 12 replicate populations tracked over about 10,000 generations. Fitness and cell size of populations increased over time. Pattern of change interesting: steplike. Why is it steplike? 5.25 Step-like pattern results when a new mutation occurs and sweeps through the population as mutant bacteria out-reproduce competitors. Remember, without mutation evolution would eventually cease. Mutation is ultimate source of genetic variation. Most mutations are deleterious and natural selection acts to remove them from population. Deleterious alleles persist, however, because mutation continually produces them. When rate at which deleterious alleles being eliminated is equal to their rate of production by mutation we have mutation- selection balance. Equilibrium frequency of deleterious allele q = square root of µ/s where µ is mutation rate and s is the selection coefficient (measure of strength of selection against allele; ranges from 0 to 1). See Box 7.8 for derivation of equation. Equation makes intuitive sense. If s is small (mutation only mildly deleterious) and µ (mutation rate) is high than q (allele frequency) will also be relatively high. If s is large and µ is low, than q will be low too. Spinal muscular atrophy is a generally lethal condition caused by a mutation on chromosome 5. Selection coefficient estimated at 0.9. Deleterious allele frequency about 0.01 in Caucasians. Inserting above numbers into equation and solving for µ get estimated mutation rate of 0.9 X 10-4 Observed mutation rate is about 1.1 X10-4, very close agreement in estimates. High frequency of allele accounted for by observed mutation rate. Cystic fibrosis is caused by a loss of function mutation at locus on chromosome 7 that codes for CFTR protein (cell surface protein in lungs and intestines). Major function of protein is to destroy Pseudomonas aeruginosa bacteria. Bacterium causes severe lung infections in CF patients. Very strong selection against CF alleles, but CF frequency about 0.02 in Europeans. Can mutation rate account for high frequency? Assume selection coefficient (s) of 1 and q = 0.02. Estimate mutation rate µ is 4.0 X 10-4 But actual mutation rate is only 6.7 X 10-7 Is there an alternative explanation? May be heterozygote advantage. Pier et al. (1998) hypothesized CF heterozygotes may be resistant to typhoid fever. Typhoid fever caused by Salmonella typhi bacteria. Bacteria infiltrate gut by crossing epithelial cells. Hypothesized that S. typhi bacteria may use CFTR protein to enter cells. If so, CF-heterozygotes should be less vulnerable to S. typhi because their gut epithilial cells have fewer CFTR proteins on cell surface. Experimental test. Produced mouse cells with three different CFTR genotypes CFTR homozygote (wild type) CFTR/F508 heterozygote (F508 most common CF mutant allele) F508/F508 homozygote Exposed cells to S. typhi bacteria. Measured number of bacteria that entered cells. Clear results Fig 5.27a F508/F508 homozygote almost totally resistant to S. typhi. Wild type homozygote highly vulnerable Heterozygote contained 86% fewer bacteria than wild type. Further support for idea F508 provides resistance to typhoid provided by positive relationship between F508 allele frequency in generation after typhoid outbreak and severity of the outbreak. Fig 5.27b Data from 11 European countries Another assumption of Hardy-Weinberg is that random mating takes place. The most common form of non-random mating is inbreeding which occurs when close relatives mate with each other. Most extreme form of inbreeding is self fertilization. In a population of self fertilizing organisms all homozygotes will produce only homozygous offspring. Heterozygotes will produce offspring 50% of which will be homozygous and 50% heterozygous. How will this affect the frequency of heterozygotes each generation? In each generation the proportion of heterozygous individuals in the population will decline. Because inbreeding produces an excess of homozygotes in a population deviations from Hardy-Weinberg expectations can be used to detect such inbreeding in wild populations. Sea otters once abundant along the west coast of the U.S were almost wiped out by fur hunters in the 18th and 19th centuries. California population reached a low of 50 individuals (now over 1,500). As a result of this bottleneck the population has less genetic diversity than it once had. Population still at a low density and Lidicker and McCollum (1997) investigated whether this resulted in inbreeding. Determined genotypes of 33 otters for PAP locus, which has two alleles S (slow) and F (fast) The genotypes of the 33 otters were: › SS 16 › SF 7 › FF 10 This gives approximate allele frequencies of S= 0.6 and F = 0.4 If otter population in H-W equilibrium, genotype frequencies should be › SS = 0.6* 0.6 = 0.36 › SF =2*0.6*0.4 = 0.48 › FF = 0.4*0.4 = 0.16 However actual frequencies were: › SS= 0.485, SF= 0.212, FF =0.303 There are more homozygotes and fewer heterozygotes than expected for a random mating population. Having considered alternative explanations for deficit of heterozygotes Lidicker and McCollum (1997) concluded that sea otter populations show evidence of inbreedng. Self-fertilization and sibling mating most extreme forms of inbreeding, but matings between more distant relatives (e.g. cousins) has same effect on frequency of homozygotes, but rate is slower. F = Coefficient of inbreeding: probability that two alleles in an individual are identical by descent (both alleles are copies of a particular ancestor’s allele in some previous generation). F increases as relatedness increases. If we compare heterozygosity of inbred population Hf with that of a random mating population Ho relationship is Hf = Ho (1-F) Anytime F>0 frequency of heterozygotes is reduced and frequency of homozygotes naturally increases. Calculating F. Need to use pedigree diagrams. Example: Female is daughter of two halfsiblings. Two ways female could receive alleles that are identical by descent. Male Female Female Male Male Fig 6.27a Half-sibling mating Fig 6.27b Total probability of scenario is 1/16 + 1/16 = 1/8. Inbreeding increases frequency of homozygotes and thus the probability that deleterious alleles are visible to selection. In humans, children of first cousins have higher mortality rates than children of unrelated individuals. Each dot on graph represents mortality rates for a human population. Fig 6.28 Mortality rate for children of cousins consistently about 4% higher than rate for children of non-relatives. In a study of 2760 individuals from 25 Croatian islands Rudan et al. found a strong positive relationship between high blood pressure and the inbreeding coefficent. Inbreeding depression also documented in studies of wild animals. E.g. Great Tit. Two studies show that survival of inbred nestlings is lower than that of outbred individuals and that hatching success of inbred eggs is lower than that of outbred eggs. Fig. 6.30