Presentation
advertisement

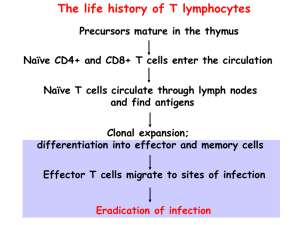
The life history of T lymphocytes Precursors mature in the thymus Naïve CD4+ and CD8+ T cells enter the circulation Naïve T cells circulate through lymph nodes and find antigens Clonal expansion; differentiation into effector and memory cells Effector T cells migrate to sites of infection Eradication of infection Lecture outline • Properties and functions of subsets of CD4+ effector T cells; functions of cytokines • Activation and functions of CTLs • Cell-mediated immunity: T cell-mediated defense against intracellular microbes – T cell-mediated macrophage activation Role of T cells in host defense Functions of CD4 helper T cells, the master controllers of immune responses, are mediated by cytokines Discovery of Th1 and Th2 subsets • Immune responses to mycobacteria and helminths are very different but CD4+ T cells are required for both – How can the “same” CD4+ T cells trigger such distinct reactions? Discovery of Th1 and Th2 subsets • Immune responses to mycobacteria and helminths are very different but CD4+ T cells are required for both – How can the “same” CD4+ T cells trigger such distinct reactions? • Hypothesis: CD4+ T cells consist of subpopulations that mediate different responses • Identification of mouse CD4+ Th1, Th2 clones that produce distinct cytokines The discovery of the Th17 subset • The first two subsets were identified on the basis of distinct cytokine profiles and were called type 1 and type 2 helper cells (Th1 and Th2) • Many inflammatory diseases (mouse models first) thought to be caused by Th1 cells were not prevented by eliminating Th1 cells or their cytokines • Led to the discovery of the Th17 subset (annoying nomenclature!) CD4+ TH subsets CD4+ T cell subsets: definitions and general properties • Populations of CD4+ T cells that make restricted and non-overlapping sets of cytokines – Early after activation, T cells can produce multiple cytokines – Progressive activation leads to “polarization”: production of selected cytokines • Distinct functions, migration properties, roles in disease Helper T cell subsets • CD4+ helper T cells orchestrate specialized immune responses to different types of microbes • The development of different Th subsets is driven by cytokines produced by APCs and other cells when naïve CD4 cells are being activated – Each subset is induced by the types of microbes that subset is designed to combat • Once a subset is generated, it makes cytokines that induce more of the same and shut off the others --> polarization of the immune response towards increasing specialization (a feature of adaptive immunity) Effector functions of TH1 Cells TH1 cells stimulate phagocyte-dependent host defense, the defense mechanism that works against most bacteria and viruses that enter tissues and are ingested by phagocytes Development of TH1 cells Th1 cells develop in response to microbes that activate dendritic cells and macrophages (most ingested bacteria and viruses). Effector functions of TH2 Cells Th2 cells combat helminths, provide defense at mucosal barriers (“barrier immunity”), and are involved in allergic reactions. Development of TH2 cells Th2 cells develop in response to organisms that induce the local production of IL-4 and do not activate macrophages and dendritic cells strongly. Effector functions of TH17 Cells Th17 cells combat extracellular bacteria and fungi. These microbes activate DCs to produce Th17-inducing cytokines. Th17 cells are also involved in many inflammatory diseases. Genetic proof for the importance of different T cell subsets in humans • Mutations affecting IL-12/IFN-g cytokines or receptors defective Th1 responses atypical mycobacterial infections • Mutations affecting Th17 development or IL-17 mucocutaneous candidiasis and bacterial abscesses (“Job’s syndrome”) 17 Roles of T cell subsets in disease • Th1: autoimmune and inflammatory diseases (IBD?, MS?, RA?); tissue damage in infections (e.g. Tb) – Activation of macrophages, CTL responses; production of injurious antibodies • Th2: allergies (e.g. asthma) – Stimulation of IgE responses, activation of eosinophils • Th17: inflammatory diseases (MS, IBD, RA, psoriasis) – Recruitment of leukocytes (inflammation) The balance of Th1 and Th2 responses determines the outcome of some infections Role of CD40L in functions of CD4 cells Mutations of CD40L (or CD40) are the cause of a human disease associated with defects in antibody responses and CMI Requirement for cell-cell contact ensures T cells recognize macrophages or B cells with the relevant antigen Cytotoxic (cytolytic) T lymphocytes (CTLs) • CD8+ CD4- cells that recognize class I MHCassociated peptides derived from cytoplasmic protein antigens in any nucleated cell • Effector functions: – Killing of infected cells (microbes in cytoplasm), tumor cells (tumor antigens in cytoplasm) – Secretion of IFN-g --> activation of macrophages (when microbes escape into cytoplasm and antigens enter class I MHC pathway) Mechanisms of killing of infected cells by CD8+ CTLs CTLs “inject” their granule contents into target cells; major granule contents are caspases, enzymes that induce apoptotic death of the target cells, i.e. help the target cells to commit suicide Cell-mediated immunity against the intracellular bacterium Listeria monocytogenes In defense against intracellular microbes,T cells provide specificity and activate phagocytes, phagocytes kill the bacteria Cell-mediated immunity (CMI) • Defense against intracellular microbes • Mediated by T lymphocytes – CD4+ T cells activate phagocytes (macrophages and neutrophils), eosinophils – CD8+ T cells kill infected cells, activate macrophages – T cell-mediated leukocyte recruitment and macrophage activation also cause delayed type hypersensitivity (DTH), which may have injurious effects Stages in the development of T cell responses: induction Stages in the development of T cell responses: effector phase Activation of macrophages by T cells T cells (via CD40L, IFNg) stimulate production of microbicidal substances in macrophages -- make phagocytes better able to kill what they eat. Functions of activated macrophages in CMI Macrophage response Role in CMI Production of reactive oxygen species, nitric oxide, lysosomal enzymes Killing of microbes in phagolysosomes (main effector function) Secretion of cytokines (TNF, IL-1, chemokines, others) Leukocyte recruitment (inflammation) Increased expression of B7 costimulators, MHC molecules Increased T cell activation (adaptive immunity) Macrophages are activated by TLR recognition of microbes as part of innate immunity; T cells activate them more Classical and alternative macrophage activation Cooperation of CD4 and CD8 cells in host defense Phagocytes eat microbes into vesicles – CD4+ T cells help phagocytes to kill microbes in vesicles Microbes evolve to escape from vesicles into cytoplasm (where they cannot be killed by microbicidal mechanisms of phagocytes) – CD8+ CTLs destroy infected cells and eradicate infection The life history of T lymphocytes Precursors mature in the thymus Naïve CD4+ and CD8+ T cells enter the circulation Naïve T cells circulate through lymph nodes and find antigens Clonal expansion; differentiation into effector and memory cells Effector T cells migrate to sites of infection Eradication of infection