Presentazione standard di PowerPoint
advertisement
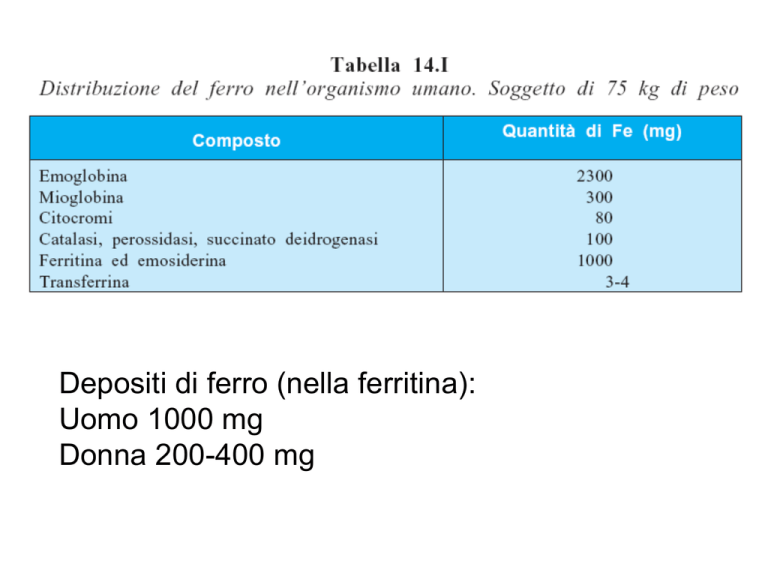
Depositi di ferro (nella ferritina): Uomo 1000 mg Donna 200-400 mg Transferrins are iron-binding blood plasma glycoproteins that control the level of free iron in biological fluids. Transferrin glycoproteins bind iron very tightly, but reversibly. Although iron bound to transferrin is less than 0.1% (4 mg) of the total body iron, it is the most important iron pool, with the highest rate of turnover (25 mg/24 h). Transferrin has a molecular weight of around 80 kDa and contains two specific high-affinity Fe(III) binding sites. The affinity of transferrin for Fe(III) is extremely high at pH 7.4 but decreases progressively with decreasing pH below neutrality. When not bound to iron, it is known as "apotransferrin" . In humans, transferrin consists of a polypeptide chain containing 679 amino acids. The protein is composed of alpha helices and beta sheets to form two domains. The N- and C- terminal sequences are represented by globular lobes and between the two lobes is an ironbinding site. When a transferrin protein loaded with iron encounters a transferrin receptor on the surface of a cell (e.g., to erythroid precursors in the bone marrow), it binds to it and, as a consequence, is transported into the cell in a vesicle by receptor-mediated endocytosis. The pH of the vesicle is reduced by hydrogen ion pumps (H+ ATPases) to about 5.5, causing transferrin to release its iron ions. The receptor (with its ligand, transferrin, bound) is then transported through the endocytic cycle back to the cell surface, ready for another round of iron uptake. Each transferrin molecule has the ability to carry two iron ions in the ferric form (Fe3+). Ferritins are complex twenty-four subunit heteropolymers of H (for heavy) and L (for light) protein subunits. The subunits of the ferritin molecule form a sphere with a central cavity in which up to 4500 atoms of crystalline iron are stored. The ranges for ferritin can vary between laboratories but are usually between 30–400 ng/mL (=μg/L) for males, and 15–200 ng/mL (=μg/L) for females. Serum Iron (SI): Men: 65 to 176 μg/dL Women: 50 to 170 μg/dL TIBC (total iron binding capacity) : 240–450 μg/dL Transferrin saturation: 20–50% μg/dL = micrograms per deciliter Normal reference ranges for transferrin are 204–360 mg/dL. Iron in food is present as ferric iron or as heme. Currently, there are two prevailing hypotheses explaining the mechanisms of iron uptake by enterocytes; first, heme is taken up by receptor mediated endocytosis; secondly, the recent discovery of a heme transporter (PCFT/HCP1) that may have the capability of transferring heme from the small intestinal lumen directly into the cytoplasm Non-haem iron requires to be converted in ferrous iron by the apical ferric reductase duodenal cytochrome B although the physiological significance of this pathway is the subject of continued debate. Following the reduction, iron crosses into the cytoplasm via an apical iron transporter, DMT1, divalent metal transporter 1. Specialised mechanism for exporting iron to plasma: the iron exporter ferroportin. Ferroportin needs copper-ferroxidases to release iron to plasma transferrin, namely hephaestin in duodenal cells and ceruloplasmin in hepatocytes, and macrophages The 25-amino acid peptide of hepcidin is secreted by the liver, which seems to be the "master regulator" of iron metabolism. Hepcidin inhibits iron transport by binding to the iron channel ferroportin, which is located on the basolateral surface of gut enterocytes and the plasma membrane of reticuloendothelial cells (macrophages). By inhibiting ferroportin, hepcidin prevents enterocytes of the intestines from secreting iron into the hepatic portal system, thereby functionally reducing iron absorption. The liver peptide hepcidin regulates intestinal iron absorption and iron release from storage cells by binding ferroportin causing its internalization and degradation, thus exerting a general inhibitory effect on iron release in the body. Animal models clarified the role of hepcidin as hepcidin knock-out mice developed massive iron overload and hepcidin overexpression induced iron deficiency. In physiological conditions, hepcidin production is tightly regulated in response to signals released from other organs, prevalently from bone marrow (erythroid regulator) and the iron stores (store regulator). Hepcidin levels increase in iron overload in order to limit iron absorption and are reduced up to undetectable levels in iron deficient erythropoiesis, either dependent from decreased iron supply or increased erythroid iron requirement, to allow iron acquisition Ferroportin acts under the control of hepcidin and this interaction can explain the systemic regulation of iron metabolism. Interestingly, it has been shown that hepcidin-induced internalization of ferroportin requires binding and cooperative interaction with Janus kinase (Jak2). Hepcidin binding to ferroportin results in the phosphorylation of ferroportin, a step necessary for its internalization by clathrin-coated pits and the kinase responsible for the phosphorylation is Jak2. Studies on genetic disorders of iron metabolism and of corresponding animal models have identified the hemochromatosis proteins (HFE, TFR2 and HJV) as the iron-dependent regulators of hepcidin expression. Patients affected by hemochromatosis have a defective synthesis of hepcidin that is absent in JH, reduced in type 3 HH due to TFR2 mutation or inadequate to the amount of iron overload in classical HH. L'emocromatosi (greco hàima sangue, e chroma, -atos colore), in passato chiamato anche diabete bronzino, è una malattia metabolica genetica dovuta all'accumulo di notevoli quantità di ferro in diversi organi e tessuti quali: fegato, pancreas, cute, cuore ed alcune ghiandole endocrine. L'assorbimento intestinale del ferro negli alimenti è aumentato. Ciò è causato, nella maggior parte dei casi, dalla mutazione genetica del gene HFE, localizzato sul cromosoma 6 Unusually, the official gene symbol (HFE for High Iron Fe) is not an abbreviation of the official name (hemochromatosis). Tra le popolazioni nord-europee il tasso di eterozigosi è del 10% circa, con lo 0,3-0,5% di omozigosi. Le manifestazioni sono 5-10 volte più frequente negli uomini, in quanto l'espressione genica della malattia è influenzata da fattori esterni quali l'introito di ferro con la dieta, le perdite ematiche associate a mestruazioni e gravidanza, le donazioni di sangue. L'esordio avviene comunemente tra i 40 ed i 60 anni; i soggetti più giovani possono essere identificati tramite screening. Hemojuvelin (HJV/RGMc/HFE2) is a membranebound and soluble protein in mammals that is responsible for the iron overload condition known as juvenile hemochromatosis in humans, a severe form of hemochromatosis. In humans, the hemojuvelin protein is encoded by the HFE2 gene.