RNA
advertisement
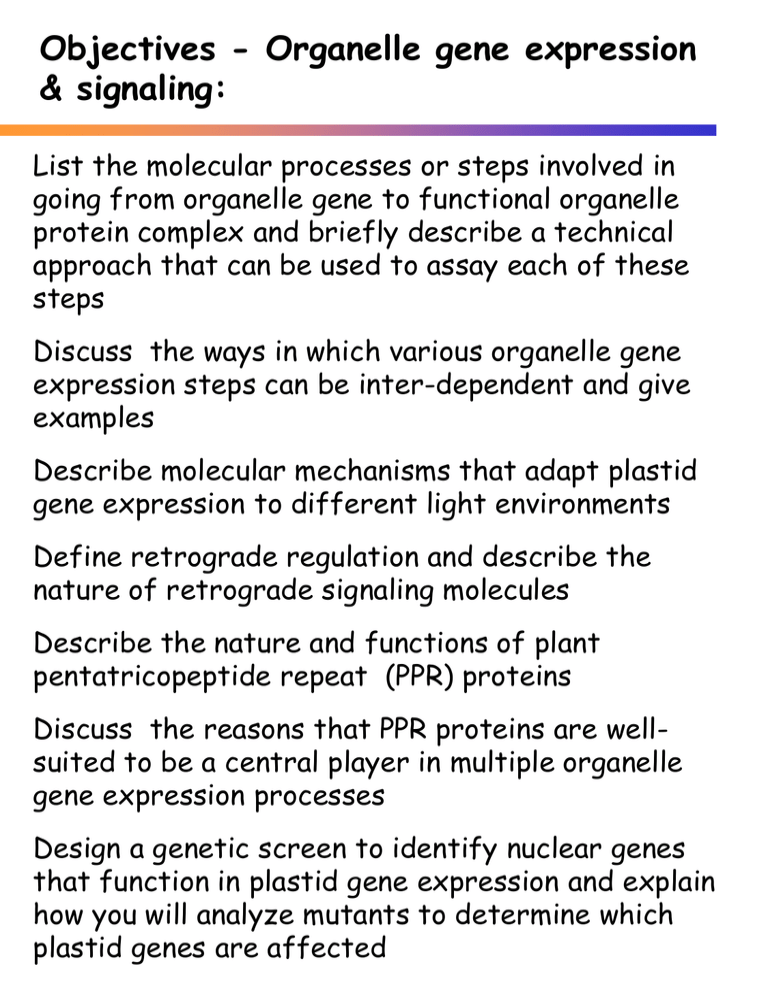
Objectives - Organelle gene expression & signaling: List the molecular processes or steps involved in going from organelle gene to functional organelle protein complex and briefly describe a technical approach that can be used to assay each of these steps Discuss the ways in which various organelle gene expression steps can be inter-dependent and give examples Describe molecular mechanisms that adapt plastid gene expression to different light environments Define retrograde regulation and describe the nature of retrograde signaling molecules Describe the nature and functions of plant pentatricopeptide repeat (PPR) proteins Discuss the reasons that PPR proteins are wellsuited to be a central player in multiple organelle gene expression processes Design a genetic screen to identify nuclear genes that function in plastid gene expression and explain how you will analyze mutants to determine which plastid genes are affected What are the processes needed to take us from gene to fully functional, multi-subunit, organelle protein complex? Plastid gene expression overview Translation (del Campo Gene Reg & Syst Biol 3:31) Organelle DNA copy number can influence gene expression levels 1 – Consider RUBISCO – the most abundant protein on earth! 2 – Mitochondrial orf239 in Phaseolus vulgaris • • • • • cytoplasmic male sterility (CMS) gene locates on a subgenomic molecule high copy number > CMS reduced copy number > pollen fertility copy number mediated by nuclear gene – Fr (Mackenzie and Chase Plant Cell 2:905) RNA Polymerases and promoters Polymerase Subunits Consensus promoter αββ’ β’’& σ 70 -35/-10 GTGTTGACA/TATAA TG Plastid – encoded (PEP) αββ’ & nuclearencoded σ specificity -35/-10 -TTGACA/TATAAT Phage T7 single core no σ overlaps initiation ATACGACTCACTATA GGGAGA Nuclear encoded plastid (NEP) T7-like core & +/- specificity factor overlaps initiation ATAGAAT A/G AA Nuclear – encoded mit T7-like core & +/- specificity factor Bacterial overlaps initiation CRTA G/T Differential plastid gene expression based upon recognition of distinct promoters by NEP and PEP Most plastid genes have promoters for both polymerases Genes encoding expression machinery (e.g. rpo, rrn, rps, rpl) primarily transcribed by NEP Photosynthetic genes primarily transcribed by PEP (from Hajdukiewicz et al. EMBO J 16:4041) Organelle transcripts - initiated vs. processed 5’ ends initiated 5’ end - PPP * processed 5’ end - P PPP * Organelle transcripts - initiated vs. processed 5’ ends Processed transcripts 5’ mono-phosphate Substrate for ligation e.g. RNA adapter for 5’ RACE e.g. Self-ligation -> Circularization Initiated transcripts 5’ tri-phosphate Ligate only after de-phosphorylation tobacco acid pyrophosphatese (TAP) Compare 5’ RACE products +/—TAP initiated transcript –not a ligation substrate 5’PPP RNA 3’ processed or TAP-treated transcript RNA adaptor Adaptor primer RNA P cDNA PCR product 3’ 3’ Gene 3’ primer PCR products containing initiated 5’ ends appear only after TAP treatment Identification of promoters in Arabidopsis plastids + T: with tobacco acid pyrophosphatase treatment - T: no pyrophosphatase treatment g: green tissue w: white tissue (seedlings grown on spectinomycin) [Swiatecka-Hagenbruch Mol Genet Genomics 277:725] Diversity of promoters in Arabidopsis plastids [Swiatecka-Hagenbruch Mol Genet Genomics 277:725] Plasticity of promoters in Arabidopsis mitochondria +TAP - TAP [Kühn et al. Nucleic Acids Res. 33:337] Plasticity of promoters in Arabidopsis mitochondria Consensus for 20 sequences supporting initiation at A Consensus of 11 sequences supporting initiation at G [Kühn et al. Nucleic Acids Res. 33:337] Differential plastid gene expression - polymerases and sigma subunits [from Lopez-Juez and Pyke Intl Int. J Dev J. BiolDev. 49:557] [Lopez-Juez & Pyke, Biol. 49: 557 ] Multiple sigma factors of A. thaliana with different plastid promoter targets II(↑) over expression +Sig2 +Sig5 I (↓) under expression −Sig2 −Sig4 trnEYD ndhF trnV trnM psaJ psbAa constpsbDb −Sig5 LRPpsbDb −Sig6 atpBEtrnEYD psaA 2.6kbb psbAc psbA psbA psbBc psbB psbCc psbD psbDc psbHc psbNc psbTc rbcLc rrn16c rrn23c rrn5c rrn4.5c SIG2 and SIG6 are essential – knock outs are chlorophyll deficient [Lysenko, Plant Cell Rep. 26:845] Redox regulation of photosynthetic gene expression is adaptive PSI PSII PET Light II PSII most efficient PSI less efficient Additional PSI subunits needed PQ highly reduced (as in + DBMIB) Light I PSI most efficient PSII less efficient Additional PSII subunits needed PQ highly oxidized (as in + DCMU) [Surpin, Plant Cell Supplement 2002:S327] Regulation of plastid transcription through plastid redox signals PSI PSII Why do the curves for relative transcript amounts and relative transcription activity differ? What do these two things measure? Complementary changes in transcription rate and mRNA abundance for psaAB (photosystem I) and psbA (photosystem II) during acclimation to light I or light II [Pfannschmidt et al. Nature 397:625] Regulation of nuclear gene transcription through plastid redox signals PSI or PET nuclear gene promoters • Fused to GUS reporter gene • GUS activity measured in response to light changes [Pfannschmidt et al. J Biol Chem. 276:36125] Transduction pathways of photosynthetic redox signals [Pfannschmidt et al. Ann Bot 103:599] Plant organelle RNA metabolism Plant organelle genes are often cotranscribed • Plastid operons • Mitochondria – di-cistronic transcripts In contrast to prokaryotic transcripts, plant organelle transcripts: • Are processed to di or mono-cistronic transcripts • Frequently contain introns • Must undergo RNA editing Plant organelle RNA metabolism: psbB operon processing in maize Plastid operons Processed to di or mono-cistronic forms • endo- and exo-nucleases • termini stabilized by stem-loops • termini stabilized by PPR protein binding [Barkan Plant Physiol 155:1524] Plant organelle RNA metabolism: psbB operon processing in maize Plastid operons Frequently contain introns • Splicing mediated by different sub-sets of nuclear-encoded RNA binding proteins [Barkan Plant Physiol 155:1524] Plant organelle RNA metabolism: psbB operon processing in maize RNA processing factors are discovered through forward genetics!!!!!!!!!!!! • APO1, APO2 • HCF107, HCF152 • CAF1, CAF2 • RNC1 • CFM3 • WTF1 • CRP1 [Barkan Plant Physiol 155:1524] High chlorophyll fluorescence (hcf) mutants (maize and arabidopsis) Mutants in the nuclear genes required for plastid biogenesis and function hcf/hcf > pale-green, yellow, or albino seedlings; some fluoresce in the dark due to dysfunctional photosystems hcf/hcf seedlings are lethal, but in maize they grow large enough for molecular analysis [Jenkins et al. Plant Cell 9:283] Nuclear mutation crp1 Disrupts processing of the psbB operon missing in crp1/crp1 mutant seedlings: • 1.1 and 0.75 kb petB RNA • 0.75 kb petD RNA •How do we see this experimentally? [Barkan et al. EMBOJ13:3170] Nuclear mutation crp1 • Disrupts processing of the psbB-psbHpetB-petD operon Which proteins are reduced in the crp1 mutant? We saw RNA processing effects for petB & petD transcripts. Why might PSAA/B be affected? Why are ALL of the PET protein subunits missing? (Hint, there must be 50 ways to lose a protein, name two!) (Barkan et al. EMBOJ 13:3170) Nuclear mutation crp1 PET A,B,C,D protein translation studies 35S-labeled leaf proteins immunoprecipitated 35S-labeled in organello synthesized proteins immunoprecipitated Which proteins are translated in the crp1 mutant? Which are not? We saw PETA, B,C & D proteins did not accumulate in this mutant. What explains the difference between translation and accumulation? [Barkan et al. EMBOJ 13:3170] Nuclear mutation crp1 PET A,B,C,D protein translation studies petB stop codon petD start codon Secondary structures of monocistronic petD (left) and bi-cistronic petB-petD (right) transcripts Propose a model: How does and RNA processing defect interfere with protein synthesis? [Barkan et al. EMBOJ 13:3170] Inter-dependence of plant organelle gene expression steps No monocistronic petD transcripts and no PETD translation • The petD initiation codon is buried in secondary structure in the petB / petD transcript • The petD initiation codon is free of secondary structure in the monocistronic petD transcript But what about • PETB and PETC – Translated but no accumulation – What is likely mechanism here? • PETA – Not translated ! – What possible mechanisms here? CRP1 associates w/ the 5’ region of the petA transcript Immunoprecipitate CRP1 RNA-protein complexes Slot-blot and hybridize • Immunoprecipitated RNA (pellet) • Unbound RNA (supernatant) PET1 protein associates with regions 5’ of petA and 5’ of psaC Does this show direct RNA binding? [Schmitz-Linneweber et al. Plant Cell 17:2791] CRP1- RNA interactions Why is the identification of two interaction sites much more powerful than one? C – consensus RNA binging site for CRP1 based on two binding regions D - model for CRP1 protein – RNA interaction [Schmitz-Linneweber et al. Plant Cell 17:2791] CRP1 is a Pentatricopeptide repeat (PPR) protein One of the largest multigene families in plants • 441 members in arabidopsis vs 7 in humans Plastid- or mitochondria-targeted Most aspects of post-transcriptional RNA metabolism • e.g. crp1 locus in maize necessary for plastid petB / petD RNA processing •e.g. restorer-of-fertility loci for CMS in petunia, radish and rice all influence processing or stability of mitochondrial CMS gene transcripts • e.g. editing of plastid ndh gene transcripts Pentatricopeptide repeat (PPR) proteins Why so many? •? RNA editing How do they function? • Site-specific RNA binding proteins • Endo and Exonucleases • Recruit enzymatic protein complexes • Simply melt RNA structures to allow interaction with processing, splicing, translation & editing factors Pentatricopeptide repeat (PPR) proteins Motif Structure of Arabidopsis PPR Proteins • Degenerate 35 amino acid repeats • The number and order of repeats can vary in individual proteins • The number of proteins falling into each subgroup is shown [Lurin et al. Plant Cell 16:2089] Plant organelle introns Group I and Group II, defined by characteristic secondary structures and splicing mechanisms [from Gillham 1994 Organelle Genes and Genomes] Organelle introns Group II intron structural domains are the ancestor of the nuclear splicosomal RNAs splicosomal RNAs [from Gillham 1994 Organelle Genes and Genomes] Organelle introns In land plants almost all are group II • Spoke-and-wheel structure • Necessary for splicing • Some fungal group IIs self-splice in vitro • RNA &/or protein factors required in vivo e.g. maize nuclear genes crs1 & crs2 encode proteins required for splicing •Genome rearrangements have split some group II introns Require trans-splicing Spoke-and-wheel structure can be assembled from separate transcripts! Organelle introns How do we see whether introns are spliced or not? There are lots of ways! Reverse transcribe + PCR (RT-PCR) DNA/ un-spliced RNA <R 3’ cDNA 5’ <R 3’ PCR 5’ F> spliced RNA cDNA PCR 5’ <R 3’ 5’ F> <R 3’ Others you may see: • Ribonuclease protection • Poison primer RT-PCR • RNA blot hybridization The maize crs1 and crs2 mutants disrupt the splicing of different group II introns rps16 intron [Jenkins et al. Plant Cell 9:283] Plant organelle intron splicing requires multiple nuclear-encoded splicing factors [Watkins et al. Plant Cell 23:1082 Trans-splicing Chlamydomonas psaA transcripts i1 5’ end i1 3’ end [Gillham 1994 Organelle Genes and Genomes] Plant organelle RNA metabolism: psbB operon processing in maize What two features confer RNA stability? For nuclear-encoded transcripts 3’ poly A stabilizes For organelle-encoded transcripts 3’ poly A tract DE-stabilizes • Also a de-stabilizing feature of bacterial transcripts • Enhances susceptibility to degradation by exonucleases [Barkan Plant Physiol 155:1524] Plant organelle RNA editing Post transcriptional enzymatic conversion of C > U, or less commonly, U > C Given a fully sequenced organelle genome, how would the RNA editing process be detected? genomic coding strand 5’ ....... ACG..... unedited RNA 5’ ....... ACG..... edited RNA 5’ ....... AUG.... edited cDNA 5’ ....... ATG..... Occurs in plastids and plant mitochondria • many more mitochondrial sites Primarily in coding sequences •conserves predicted protein Creates initiation codons ACG > AUG Creates termination codons CGA > UGA Removes termination codons UGA > CGA Changes amino acid coding CCA > CUA (P > L) Silent edits CTT > CTC (L > L) Plant organelle RNA editing Edit sites within the same gene vary among species • An edit site in one species may be “pre-edited” (correctly encoded in the genomic sequence) of another species • e.g. plastid psbL gene initiation codon: maize ATGACA..... tobacco ACGACA..... must be edited to AUG (RNA) = ATG (cDNA) for translation initiation codon Evolution of plant organelle RNA editing Not in algae Observed in every land plant lineage except Marchantiid liverworts [Knoop , Curr Genet 46:123] RNA editing improves evolutionary conservation Table 1. Evolutionary conserved amino acid residues changed by C-to-U editing in ribosomal protein S12 (RPS12) of plant mitochondria Amino acid residues encoded by unedited and edited maize mitochondrial transcripts compared to amino acid residues in RPS12 polypeptides from other taxa [Mulligan and Maliga (1998) pp.153-161 In A look beyond transcription J Bailey-Serres and DR Gallie (eds) ASPB] RNA editing by enzymatic de-amination 32P UTP V 32P CTP > 32P CTP [Rajasekhar and Mulligan Plant Cell 5:1843] [Russell, 1995, Genetics] Short 5’ flanking sequences define plant organelle RNA editing sites [from Mulligan and Maliga (1998) pp.153-161 In A look beyond transcription J Bailey-Serres and DR Gallie (eds) ASPB] RNA editing – genetic analysis defines a trans-acting factor [from Kotera et al. Nature 433:326] Genetic analysis defines a PPRmotif RNA editing factor [from Kotera et al. Nature 433:326] Genetic analysis defines a PPRmotif RNA editing factor The immunoblots implicating crr4 in NDH complex biogenesis showed loss of the NDHH subunit, but the affected RNA editing site is in the ndhD transcript. What are some explanations for these observations? [from Kotera et al. Nature 433:326] Translation of organelle genes A significant regulatory process in plastid gene expression light-regulated chloroplast protein accumulation increases 50-100 fold w/out changes in mRNA accumulation 5’ UTR is key in regulating translation ~ 1/2 of plastid transcripts have a 5’ Shine-Delgarno sequence (GGAG) homologous to small subunit rRNA in this region nuclear-encoded translation factors bind 5’ untranslated region (UTR) (and in some cases also the 3’ UTR) Translation of organelle genes Regulation of plastid gene translation by light - mediated by pH, ADP, redox signals e.g. translation of PSII D1 (PSBA) in Chlamydomonas • Accumulation of PSBA increased in light • No change in steady-state level of mRNA • Site-directed mutagenesis of 5’ UTR 5’ SD sequence 5’ stem-loop region Required for translation • 5’UTR binding proteins identified Binding increased 10X in the light Reduced thioredoxin required for binding Binding abolished by oxidation Binding decreased by ADP-dependent phosphorylation (ADP accumulates in the dark) The details of this mechanism are NOT conserved in angiosperms Redox regulation of PSBA protein synthesis in Chlamydomonas [Pfannschmit (2003) Trends Plant Sci 8:33] Translation of organelle genes- PPR protein RNA re-modeling enhances ATPH translation Translation of organelle genes Control by Epistasy of Synthesis (CES) Regulation of protein synthesis by presence or absence of assembly partners e.g. Down-regulation of tobacco nuclear rbcS gene by antisense •Decreased translation of rbcL in plastid e.g. Chlamydomonas plastid cytochrome f (PET complex) Absent other subunits, cytochrome f cannot assemble • Unassembled cytochrome f binds to its own (petA ) 5’ UTR • Down regulates translation Organelle protein complex assembly and protein turn-over Failure to assemble a protein complex > degradation of unassembled subunits Assembly dependent upon availability of all subunits and co-factors Plastids contain several proteases that are homologues of bacterial proteases o Functions in protein turn-over o ? Protease independent chaperone functions (as seen in bacteria) Bacterial – type proteases in plastids Protease Location and Function in plastid ClpP/ClpC stroma ATP-dependent serine protease degrades mis-targeted proteins and cytb6/f subunits FtsH stromal face of thylakoid membranes membrane-bound, ATPdependent metalloprotease DegP serine heat-shock protease degrades photo-damaged PSI protein D1 from stromal side lumenal side of thylakoid membranes degrades photo-damaged PSI protein D1 from lumen side