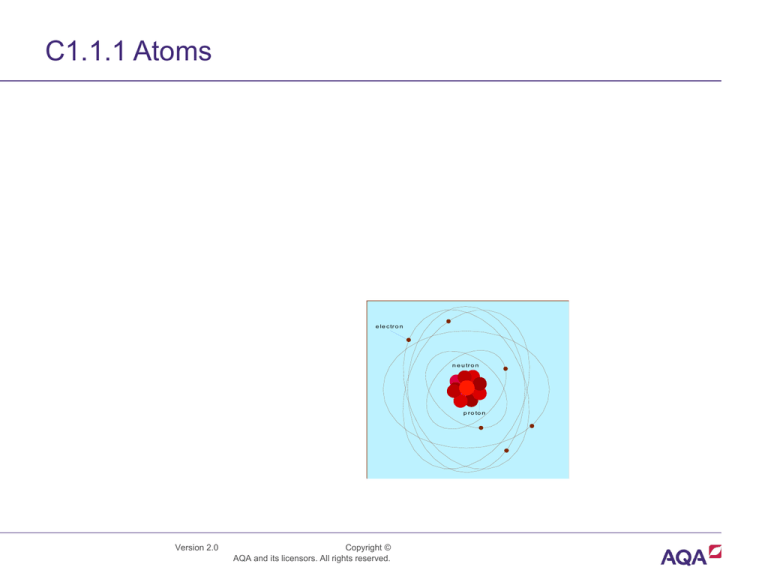
C1.1.1 Atoms
e le c tr o n
n e u tr o n
p r o to n
Version 2.0
Copyright ©
AQA and its licensors. All rights reserved.
Using Exam pro items to support successful
outcomes
• Learners will be able to test their progress against learning
outcomes using questions from past AQA GCSE questions
Version 2.0
Copyright © AQA and its
licensors. All rights reserved.
Foundation C1 Jan 12, 1
Version 2.0
Copyright © AQA and its
licensors. All rights reserved.
Complete the table to show the name and charge of each
type of particle in the carbon atom
1.
Name of Particle
Charge
Proton
Neutron
0
-1
Version 2.0
Copyright © AQA and its
licensors. All rights reserved.
Draw a ring round the correct answer to complete the
sentence
2.
3.
4.
Version 2.0
Copyright © AQA and its
licensors. All rights reserved.
Complete the sentence
5. Gold and carbon have different properties because gold is a
metal and carbon is a ………………………..
The data in the table shows some information about 3 metals
in a gold ring
Version 2.0
Copyright © AQA and its
licensors. All rights reserved.
Draw one line from each question to the correct
answer
Version 2.0
Copyright © AQA and its
licensors. All rights reserved.
Mark scheme- Foundation- Total 9 marks
Question
Answers
Extra Information
Mark
1
+1/+
Do not accept 1 without
the +
1
Electron
Allow phonetic spelling
1
2
elements
1
3
Soft
1
4
An alloy
1
5
Non- metal
1
6
One mark for each
correct link
Extra lines lose the
mark
Version 2.0
Copyright © AQA and its
licensors. All rights reserved.
3
Higher Questions C1 Jan 12 , 1
1.
Version 2.0
Copyright © AQA and its
licensors. All rights reserved.
2.
A gold atom has an atomic number of 79 and a mass number
of 197.
Complete the table to show the name and number of each
sub-atomic particle in this gold atom
Name
Number
Proton
79
Electron
Version 2.0
Copyright © AQA and its
licensors. All rights reserved.
3. The bar chart shows the composition of
this gold ring
Give the percentage of the
metals in this gold ring.
a) Silver is ……….%
b) Copper is …… %
This gold ring is not
made from 100% gold.
c) Give two reasons why.
other two metals iu
why.
Version 2.0
Copyright © AQA and its
licensors. All rights reserved.
Mark scheme- Higher- Total 7 marks
Question
Answers
Extra Information
Mark
1
2,4
Allow electrons in any
position on correct shells
1
2
(electron) 79
Neutron
118
Allow phonetic spelling
1
1
1
3a+ 3b
16 and 9
In this order
1
3c
An two from
• (100%/pure) gold is soft
• (alloyed) to make the
metal hard(er)
• Gold is expensive or
alloy is less expensive
Ignore reasons about
colour/lustre/corrosion/rarity
Allow layers can slide in pure
gold
Ignore just ‘the ring is an alloy’
Allow (alloyed) to stop the
layers sliding
Allow ( alloyed) to make the
metal strong
2
Version 2.0
Copyright © AQA and its
licensors. All rights reserved.