Chapter 11 - Materials Science
advertisement

Lec 26
26/03/2014
Plastic deformation
and
creep
in
crystalline materials
Chap. 11
Mechanical Properties of Materials
Stiffness
Resistance to elastic
deformation
Young’s
modulus
Strength
Resistance to plastic
deformation
Yield
stress
Toughness Resistance to fracture
Energy to
fracture
ductility
Strain to
fracture
Ability to deform
plastically
Uniaxial Tensile Test (Experiment 6)
Gauge
length
specimen
Result of a uniaxial tensile test
Ultimate
tensile
strength
(Engineering stress)
necking
UTS
Yield
strength
y
STRENGTH
Yield
point
break
Area = Toughness
STIFFNESS
Slope = Young’s
modulus (Y)
DUCTILITY
f (strain to fracture)
(engineering strain)
If there is a smooth
transition from elastic
to plastic region (no
distinct yield point)
then 0.2 % offset proof
stress is used
During uniaxial tensile test the length of the specimen is
continually increasing and the cross-sectional area is
decreasing.
True stress ≠ Engineering stress (=F/A0)
True strain ≠ Engineering strain (=L/L0)
True stress
P
T
Ai
True incremental strain
Ai = instantaneous area
Eqn. 11.3
dL
d T
L
L
True strain
dL
L
T
ln
L
L0
L0
Eqn. 11.4
T K
K
Strength coefficient
n
work hardening exponent
n
T
Eqn. 11.5
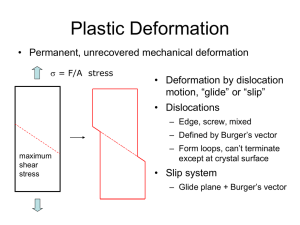
What happens
during plastic
deformation?
• Externally, permanent shape change
begins at y
• Internally, what happens?
What happens to crystal structure after
plastic deformation?
Plastic
Deformation
?
Some Possible answers
Remains the
Changes to
Becomes random
same
another
or
crystal
amorphous
structure
How Do We Decide?
X-ray diffraction
No change in crystal structure!
No change in internal crystal structure but
change in external shape!!
How does the microstructure of polycrystal
changes during plastic deformation?
EXPERIMENT 5
Comparison of undeformed Cu and deformed Cu
Slip Lines
Before Deformation
After Deformation
Callister
Slip lines in the
microstructure of
plastically deformed
Cu
Experiment 5
Slip
Slip Planes, Slip Directions, Slip
Systems
Slip Plane:
Crystallographic planes
Slip Direction: Crystallographic direction
Slip System:
A combination of a slip
plane and a slip direction
Lec 27
28/03/2014
Slip Systems in Metallic Crystals
Crystal
Slip
Plane
Slip
Direction
Slip
Systems
FCC
{111}
(4 planes)
<110>
4x3=12
(3 per plane)
BCC
{110}
(6 planes)
<111>
6x2=12
(2 per plane)
HCP
{001}
(1 plane)
<100>
3x1=3
(3 per plane)
Why slip planes are usually close packed
planes?
Why slip directions are close-packed
directions?
Slip Systems in FCC Crystal
(111)
z
y
x
Tensile vs Shear Stress
• Plastic deformation takes place by slip
• Slip requires shear stress
• Then, how does plastic deformation
take place during a tensile test?
: Applied tensile stress
N: Slip plane normal
N
D: Slip direction
1
2
D
F1: angle between and N
F2 =angle between and D
Is there any shear stress on the
slip plane in the slip direction
due to the applied tensile
stress?
Resolved Shear stress
F
= F/ A
Area=A
FD = F cos 2
N
1
2
D
As = A cos 1
RSS
Area = As
FD
AS
F cos2
A
cos1
F
cos1 cos2
A
F
RSS cos1 cos2
F
F
No resolved
shear stress
on planes
parallel or
perpendicular
to the stress
axis
F
cos 2 = 0
F
cos 1 = 0
Plastic deformation recap
No change in crystal structure:
slip
twinning
Slip takes place on slip systems (plane + direction)
Slip planes usually close-packed planes
Slip directions usually close-packed direction
Slip requires shear stress
In uniaxial tension there is a shear component
of tensile stress on the slip plane in the slip
direction:
RESOLVED SHEAR STRESS
CRITICAL RESOVED SHEAR STRESS
RSS
RSS cos1 cos2
CRSS
N
1
2
D
cos1 cos2
y
CRSS y cos1 cos2
Lec 28
01.04.2014
CRSS y cos1 cos2
If we change the direction of stress with respect to the
slip plane and the slip direction cos 1 cos 2 will change.
To maintain the equality which of the following
changes takes place?
1. CRSS changes.
2. y changes
Schmid’s Law:
CRSS is a material constant.
Anisotropy of Yield Stress
crss
y
cos1cos2
Yield stress of a single crystal depends upon the
direction of application of load
cos 1 cos 2 is called the Schmid factor
Active slip system
RSS
cos1a cos 2a
CRSS
cos1b cos 2b
y
CRSS y cos1 cos2
Slip system with
highest Schmid
factor is the
active slip system
Magnitude
of
Critical Resolved Shear Stress
Theory (Frenkel 1926)
Experiment
Potential
energy
Shear stress
CRSS
b/2
b
d
b
Critical Resolved Shear Stress
Theory Experiment
Ratio
(GPa)
(MPa)
Theory/Exp
Fe (BCC)
12
15
800
Cu (FCC)
7
0.5
14,000
Zn (HCP)
5
0.3
17,000
?
Solution
1934
E. Orowan
Michael Polanyi
Geoffrey Ingram Taylor
Solution
• Not a rigid body slip
• Part slip/ part unslipped
Slip
Not-yet-slipped
Boundary between slipped and unslipped parts
on the slip plane
Dislocation Line (One-Dimensional Defect)
Movement of an Edge Dislocation
From
W.D. Callister
Materials
Science
and Engineering
Plastic Deformation Summary
• Plastic deformation
•
Slip
slip
dislocations
• Plastic deformation requires movement of
dislocations on the slip plane
Recipe for strength?
Remove the dislocation
Stress,
MPa
Fig. 11.6
700
50
strain
Cu Whiskers tested in tension
Lec 29
02.04.2014
crystal structure changes?
No
Mechanism of deformation
slip
Nature of stress required for slip
Is there shear during tension?
Resolved shear stress
required for initiating slip
Shear stress
Resolved shear stress
Critical resolved shear stress
CRSS is independent of the direction of application of
tensile stress
Lec 29
02.04.2014
Effect of temperature on dislocation motion
Higher temperature makes the dislocation motion easier
Yield stress
F
e
W
Al2
O3
S
i
18-8 ss
Eqn. 11.14
Ni
11.15
Fig. 11.8
Cu
11.16
11.17
0
T/Tm
0.7
11.18
Recipe for strength
Remove the dislocation:
Possible but Impractical
Alternative:
Make the dislocation motion
DIFFICULT
Strengthening Mechanisms
• Strain hardening
• Grain refinement
• Solid solution hardening
• Precipitation hardening
Movement of an Edge Dislocation
A unit slip takes
place only when
the dislocation
comes out of the
crystal
During plastic deformation dislocation
density
of a crystal should go down
Experimental Result
Dislocation Density of a crystal actually goes up
Well-annealed crystal: 1010 m-2
Lightly cold-worked: 1012 m-2
Heavily cold-worked: 1016 m-2
?
Dislocation Sources
F.C. Frank and W.T. Read
Symposium
on
Plastic Deformation of Crystalline Solids
Pittsburgh, 1950
P
b
A
b
B
b
Q
b
http://zig.onera.fr/~douin/index.html
b
b
Fig. 11.9
http://zig.onera.fr/~douin/index.html
Problem 11.11
Strain Hardening or Work
hardening
y
y
Strain,
During plastic deformation dislocation density increases.
Dislocations are the cause of weakness of real crystals
Thus as a result of plastic deformation the crystal should
weaken.
However, plastic deformation increases the yield strength
of the crystal: strain hardening or work hardening
?
Strain Hardening
Dislocation against Dislocation
A dislocation in the path of other
dislocation can act as an
obstacle to the motion
of the latter
Sessile dislocation in an FCC crystal
1
[10 1 ]
2
a2 a2 a2
2
2
2
1
[1 1 0]
2
Energetically
favourable reaction
1
[0 1 1]
2
(001) not a favourable slip plane
(CRSS is high).
1
[0 1 1]
2
( 1 11)
1
[1 1 0]
2
Eqn. 11.20
(001)
Fig. 11.10
The dislocation
immobile or sessile.
1
[10 1 ]
2
[110]
(1 1 1)
Sessile dislocation a barrier to other
dislocations creating a dislocation pile-up
Sessile dislocation (barrier)
(1 1 1)
( 1 11)
Fig. 11.10
Piled up dislocations
Lec 30 04.04.2014
Empirical relation for strain hardening or
work hardening
0 A
Eq. 11.21
Is the shear stress to move a dislocation in a
crystal with dislocation density
o and A : empirical constants
Fig. 11.11
Dislocation Motion
Easy Dislocation Motion
Plastic Deformation
Easy Plastic Deformation
Weak Crystal
Difficult
Difficult
Dislocation Motion
Plastic Deformation
Strong Crystal
Grain Boundary
Grain 2
Grain1
Grain boundary
2-D Defect: Grain Boundaries
Single Crystal
No Grain Boundaries
Polycrystal
Grains of different orientations
separated by grain boundaries
Discontinuity of a slip plane
across a grain boundary
Slip plane
Dislocation
Grain Boundary
Grain Boundary Strengthening
• Slip plane discontinuity at grain
boundary
• A dislocation cannot glide across a grain
boundary
• Higher stresses required for deformation
• Finer the grains, greater the strength
Coarse Grains
Fine Grains
Grain Size
Strengthening
Hall-Petch Relation
k
y 0
D
y: yield strength
D: average grain diameter
0, k: constants
Some recent developments
The hardness of coarse-grained materials is inversely
proportional to the square root of the grain size. But as
Van Swygenhoven explains in her Perspective, at
nanometer scale grain sizes this relation no longer
holds. Atomistic simulations are providing key insights
into the structural and mechanical properties of
nanocrystalline metals, shedding light on the distinct
mechanism by which these materials deform.
Science 5 April 2002:
Vol. 296 no. 5565 pp. 66-67
POLYCRYSTALLINE MATERIALS
Grain Boundaries and Dislocations
Solid Solutions
• Mixture of two or more metals
• Solute atoms: a zero dimensional defect or
a point defect
• Two types:
– 1. Interstitial solid solution
– 2. Substitutional solid solution
Interstitial Solid Solution
Perfect Crystal
Distortion caused by a
large interstitial atom
Substitutional Solid Solution
Small solute atom
Large solute atom
Solute atom: a zero-dimensional point defect
Solid Solution
Strengthening
Solute
atoms
Strong
crystal
Strains in the
surrounding crystal
Obstacle to dislocation
motion
Alloys stronger than pure metals
200 Sn (1.51)
Be (1.12)
Matrix = Cu (r = 1.28 Å)
150
(Values in parenthesis are
atomic radius values in Å)
100
Zn (1.31)
50
0
40
20
10
30
Solute Concentration (Atom %) →
Figure: Anandh
Subramaniam
Fig 11.13
Airbus A380 to be launched on October 2007
A shop inside Airbus A380
Alfred Wilm’s Laboratory
1906-1909
Steels harden by quenching
Why not harden Al alloys also
by quenching?
Wilm’s Plan for
hardening Al4%Cu alloy
T
550ºC
Hold
Check
hardness
One of the greatest
technological
achievements of 20th
century
Sorry! No
increase in
hardness.
time
Eureka !
Hardness
has
Increased !!
Hardness increases as a function of time: AGE
HARDENING
Property = f (microstructure)
Wilm checked the microstructure of
his age-hardened alloys.
Result:
NO CHANGE in the
microstructure !!
Lec 31
Creep
09/04/2014
Lec 32
11/01.2014
Peak hardness
Hardness
As- quenched
hardness
time
Hardness initially increases: age hardening
Attains a peak value
Decreases subsequently: Overaging
Tsolvus
: solid
solution of
Cu in FCC Al
+
: intermetallic
compound CuAl2
4
supersaturated
saturated
FCC
FCC
Tetragonal
4 wt%Cu
0.5 wt%Cu
54 wt%Cu
+
Precipitation
of in
Stable
Tsolvus
TTT diagram of precipitation of in
unstable
+
Asquench
ed
Aging
A fine distribution of precipitates in matrix causes
hardening
Completion of precipitation corresponds to peak
hardness
As quenched
Aged
Peak
aged
-grains
-grains +
Dense
distribution
of fine
overaged
Driving force for
coarsening
/ interfacial energy
Sparse
distribution
of coarse
hardness
Aging temperature
100ºC
20ºC
180ºC
Fig. 9.15
Aging time
0.1
1
10
100
(days)
Peak hardness is less at higher aging temperature
Peak hardness is obtained in shorter time at higher
aging temperature
Stable
T
U
Tsolvus
unstable
+
180 ºC
Asquenched
100 ºC
Aging
I
100ºC
hardness
180ºC
1
20ºC
Hardness increases as as a function of time
hardness
As-quenched
hardness
time
No change in microstructure - Wilm!
Numerous fine precipitates form with time
Not visible in optical micrograph
X-Ray Diffraction (XRD)
Transmission Electron Microscopy (TEM)
Guinier-Preston Zones, 1938
“It seems justifiable at the moment to
conclude that the process of age
hardening in this alloys is associated
with the segregation of copper atoms on
the (100) planes of the crystal as
suggested by C.H. Desch in The
Chemistry of Solids, 1934”
Preston, 1938, “The Diffraction of X-rays
by Age-Hardening Aluminium Copper Alloys
Precipitation Hardening
Precipitates are obstacles to the motion of dislocation
Solute atoms
Pebbles
Precipitates
boulders
Cake with nuts
Age-hardening = Precipitation hardening
Dislocation-precipitate interaction
Dislocation can
1. Either cut through the precipitate
particles (small precipitate)
2. Or they can bypass the
precipitates
Precipitate cutting
before
after
Fig 11.14 a, c
Dislocation bypassing the
precipitate
b
L
Fig. 11.14 b and d
Movement of onedimensional defects called
dislocations causes plastic
deformation
Obstacles to the movement
of dislocations cause
strengthening
Strengthening Mechanisms
Name
Obstacle Type
Solid solution hardening
Solute atoms (0-D)
Strain hardening
Dislocations (1-D)
Grain refinement
Grain boundaries (2-D)
Precipitation hardening
Precipitates (3-D)
: How do glaciers move?
102
http://rmkilc.wordpress.com/
2. Electric Bulb
“Genius is one
percent
inspiration and
ninety-nine
percent
perspiration”
-T.A. Edison
Q2: How do bulbs fuse?
105
Rolls-Royce Plc
Q3: What does the Rolls-Royce plc make?
106
107
Q: What is common to all the three?
Ans: CREEP
1. Glaciers move due to creep of snow.
2. Bulbs fuse due to creep of W filament.
3. Life of jet engine depends of creep of the turbine
blades.
108
Creep
Creep is time dependent plastic deformation at
constant load or stress
Difference between normal plastic deformation
and creep ?
It is a “high temperature” deformation
T 0.4 Tm
Tm is the m.p. in K.
CREEP
Fig. 11.15
Creep Mechanisms of crystalline materials
Cross-slip
Dislocation climb
Creep
Vacancy diffusion
Grain boundary sliding
Cross-slip
In the low temperature of creep → screw dislocations can cross-slip
(by thermal activation) and can give rise to plastic strain [as f(t)]
Slip
plane 1
b
1
2
3
Dislocation climb
Edge dislocations piled up against an obstacle can climb to another slip
plane and cause plastic deformation [as f(t), in response to stress]
Rate controlling step is the diffusion of vacancies
Nabarro-Herring creep → high T → lattice diffusion
Diffusional creep
Coble creep → low T → Due to GB diffusion
In response to the applied stress vacancies preferentially move from
surfaces/interfaces (GB) of specimen transverse to the stress axis to
surfaces/interfaces parallel to the stress axis→ causing elongation
This process like dislocation creep is controlled by the diffusion of
vacancies → but diffusional does not require dislocations to operate
Flow of vacancies
Grain boundary sliding
At low temperatures the grain boundaries are ‘stronger’ than the crystal
interior and impede the motion of dislocations
Being a higher energy region, the grain boundaries melt before the crystal
interior
Above the equicohesive temperature grain boundaries are weaker than
grain and slide past one another to cause plastic deformation
Single crystal turbine blade
Pigtail: a helical
channel which
gradually
eliminates most
columnar grains
Starter: initiates columnar
grains as in Directional
Solidification (DS)
Single crystal
blade: best
creep
resistance
117
Coarser grains
-> Less grain boundaries
-> Better for creep application
Single Crystal
-> No grain boundaries
-> Best for creep application
Nanocrystalline materials
-> not good for creep applications!
118
Improvements due to blade manufacturing technique:
Show turbine blades
119
Improvements due to engineering design: Blade
cooling
Engineering Materials 1: Ashby and Jones
120
Thermal Barrier Coating (TBC)
NiCrAlY or NiCoCrAlY
Ceramic top
coat:
Yittria stabilized
Zirconia
(YSZ)
1. Low thermal
conductivity
www.matsceng.ohio-state.edu
2. High thermal
expansion
3. High M.P
Reduction in surface temp 100-300 oC
Operating temp > M.P. (~1300 oC)
121
Creep Resistant Materials
Higher operating temperatures gives better efficiency for a heat engine
High melting point → E.g. Ceramics
Dispersion hardening → ThO2 dispersed Ni (~0.9 Tm)
Creep
resistance
Solid solution strengthening
Single crystal / aligned (oriented) grains
Cost, fabrication ease, density etc. are other factors which determine
the final choice of a material
Commonly used materials → Fe, Ni, Co base alloys
Precipitation hardening (instead of dispersion hardening) is not a good
method as particles coarsen (smaller particles dissolve and larger
particles grow interparticle separation ↑)
Ni-base superalloys have Ni3(Ti,Al) precipitates which form a low
energy interface with the matrix low driving force for coarsening
Cold work cannot be used for increasing creep resistance as
recrystallization can occur which will produced strain free crystals
Fine grain size is not desirable for creep resistance →
grain boundary sliding can cause creep elongation / cavitation
► Single crystals (single crystal Ti turbine blades in gas turbine
engine have been used)
► Aligned / oriented polycrystals
No Dislocations
Ultra Strong Crystals
Whiskers
Composite Materials
Various Crystal Defects
Substitutional
solute
Stacking
fault
G-P zone
Dislocations
Interstitial
solute
Vacancy
(Diffusion)
Grain Boundary
Moral of the Story
Strength depends upon defects
Microstructure
• Structural features observed under a
microscope
– Phases and their distribution
– Grains and grain boundaries
– Twin boundaries
– Stacking faults
– Dislocations
Hierarchy of Structures
nuclear structure
Physics and
chemistry
atomic structure
1A0
crystal structure
1nm
Metallurgy and
Materials Science
Engineering: Civil,
Mechanical, etc.
microstructure
1m
macrostructure
1mm
engineering structure
1m
Real Moral of the Story
Properties depend upon
microstructure
Structure Sensitive
vs
Structure Insensitive Properties
For true understanding comprehension
of detail is imperative. Since such
detail is well nigh infinite
our knowledge is always
superficial and
imperfect.
Duc Franccois de la Rochefoucald
(1613-1680)