File
advertisement

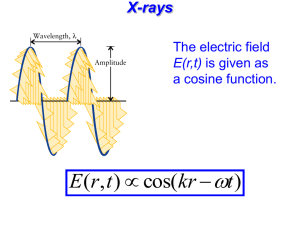
Chapter 5 Materials Characterization Lecture 1 Dr. Alagiriswamy A A (PhD., PDF) Dept. of Physics and Nanotechnology SRM University Main Campus, Ktr., SRM Nagar, Chennai, Tamilnadu 5. Characterization Techniques • 5.1 X-ray Diffraction (XRD) • 5.5 Neutron diffraction (ND) • 5.3 Electron diffraction (ED) • 5.4 X-ray fluorescence methods (XRF) • 5.5 Infrared Spectroscopy (IRS) • 5.6 Fourier Transform infrared spectroscopy (FTIR) • 5.7 Surface Raman spectroscopy (SRS) • 5.8 Ultraviolet-visible spectroscopy (UVS) • 5.9 Thermogravimetric analysis (TGA) • 5.10 Differential scanning calorimetric analysis (DSC) • 5.11 Differential thermal analysis (DTA) Characterization of Solid Samples Bulk elemental composition Dissolution step Direct solid analysis Atomic absorption ICP spectrometry Electron. microscope ICP mass spect. CHN analyser Structural Properties Surface phenomena IR spectrom. Dynamic SIMS. UV-Vis spectrom Static SIMS XRD/FESEM SEM/FESEM TEM./HRTEM SEM-EDX XPS/SAM dilatometry. AFM Excitation Source Sample Detector Why do you characterize?? Materials Characterization Materials Characterization has several main aspects Accurately measuring the physical and chemical properties of materials Providing local microscopy Accurately measuring (determining) the structure of a material - atomic level structure - microscopic level structures Background A slight history X-rays were discovered by W.C. Röentgen in 1895, and led to several uses X-ray radiography is used for creating images of light-opaque materials relies on the relationship between density of materials and absorption of xrays. Applications include a variety of medical and industrial applications. X-ray fluorescence spectrometry relies on characteristic secondary radiation emitted by materials when excited by a high-energy x-ray source and is used primarily to determine amounts of particular elements in materials. X-ray crystallography relies on the dual wave/particle nature of x-rays to discover information about the structure of crystalline materials More of interest, owing to the facts:X-rays rays – a primer X-rays: high energy (1 to 100 keV), short wavelength (1 nm to 0.05 nm) electromagnetic radiation Brehmstrahlung (or Continuum) Radiation: German for “breaking radiation”, noise that appears in the spectra due to deceleration of electrons as they strike the anode of the X-ray tube High energy + strong dependence on atomic number make X-rays a valuable (medical) diagnostic tool X-ray wavelengths are similar to the spacing of atoms in matter –not far off from the size scale of nanostructured solids non-destructive/a well-established scientific basis/mathematical analytics relatively simple demands on equipment and experimental technique X-rays can be scattered by electrons in a solid There is “life” beyond Bragg’s Law (Growing Reliance On Nanoscale Materials) Light and matter exhibit wave-particle duality Relation between wave and particle properties E h p h given by the de Broglie relations Evidence for particle properties of light , Photoelectric effect, Compton scattering Evidence for wave properties of matter Electron diffraction, interference of matter waves (electrons, neutrons, He atoms, C60 molecules) x p / 2 x Heisenberg uncertainty principle limits y p y / 2 simultaneous knowledge of conjugate Variables. This is because of intrinsic nature z p z / 2 Scattering by One Electron Oscillation direction of the electron X-ray beam The scattered energy (intensity) is: Electron Electric vector of The incident beam 2 1 e 2 I el I 0 2 2 sin r mc 2 10 Scattering by Two Electrons p q 2 s0 r 1 s The phase difference of wave 2 with respect to wave 1 is: 2r (s 0 s) 2 r S S s s0 2 1 S 2 s sin 2 sin Let the magnitudes of s0 and s = 1/λ Diffracted beams 1 and 2 have the same magnitude, but differ in phase because of the path difference p + q 1 1 How about scattering by • an atom • a row of atoms, • a plane of atoms • A unit cell • A crystal t=4 t=5 t=6 t=7 (r) t=3 t=2 t=1 2a.S t=0 t=8 +r nucleus -r (-r) April 9, 2015 12 Single molecule diffraction? The scattering by a single electron is weak, but not too weak: A crystal amplifies “the” signal by summing up the amplitudes from ~1024 electrons; Don’t you even need crystals rather than looking at single electron diffraction pattern???? The Laue method is mainly used to determine the orientation of large single crystals while radiation is reflected from, or transmitted through a fixed crystal. The diffracted beams form arrays of spots, that lie on curves on the film. The Bragg angle is fixed for every set of planes in the crystal. Each set of planes picks out and diffracts the particular wavelength from the white radiation that satisfies the Bragg law for the values of d and θ involved. For every set of crystal planes, by chance, one or more crystals will be in the correct orientation to give the correct Bragg angle to satisfy Bragg's equation. Every crystal plane is thus capable of diffraction. Each diffraction line is made up of a large number of small spots, each from a separate crystal. Each spot is so small as to give the appearance of a continuous line Neutron diffraction = h/p; = 2.860 / E1/2 •Fast moving neutrons, slow, thermal, resonance neutrons, cold, •Atomic nuclei could scatter neutrons, and emit beta particles Electron diffraction Davisson and Germer's discovery of electron diffraction Lack of surface sensitivity X-ray Fluorescence (XRF) Methods Instrumentation 1. X-ray fluorescence system utilizing an analyzing crystal The filtered primary x-rays from the tube hits the sample and produce the fluorescence radiation characteristic of the elements in the sample. This radiation passes through a collimator and the relative wavelengths are separated by the diffracting crystal. The intensity of each wavelength is determined and recorded as the synchronized detector rotates in an arc around the analyzing crystal. Schematic diagram of an X-ray fluorescence system utilizing an analyzing crystal What’s the point? We utilize the x-rays produced by the electron microprobe for many research applications. There are other techniques, similar in some ways, that are worth discussing, that utilize x-rays for secondary x-ray fluorescence. Two in particular are: XRF (X-Ray Fluorescence), where x-rays from a sealed tube are used to produce xrays by secondary fluorescence in samples of interest (traditionally a macrotechnique) Synchrotron Radiation, where electrons are accelerated in ~10s-100s meters diameter rings, and then made to produce highly focused beams of extremely intense x-rays or light, which are then fed into many different types of experiments. The benefits of secondary x-ray fluorescence include very low detection limits (10s of ppm easy in 10 seconds, no backgrounds) XRF - Features Energy dispersive X-ray fluorescence technology (ED-XRF) provides one of the simplest, most accurate and most economic analytical methods for the determination of the chemical composition of many types of materials. It is non-destructive and reliable, requires no, or very little, sample preparation and is suitable for solid, liquid and powdered samples. It can be used for a wide range of elements, from sodium (11) to uranium (92), and provides detection limits at the sub-ppm level; it can also measure concentrations of up to 100% easily and simultaneously X-ray Attenuation X-ray T A R G E T characteristic (Compton) (Rayleigh) The basics of XRF are very similar to those of EPMA—we are dealing with characteristic x-rays and continuum x-rays— with the exception that we are doing secondary fluorescence : x-ray spectroscopy of our samples using x-rays coming out of a sealed tube to excite the atoms in our specimen. The big difference is that • there is NO continuum generated in the sample (x-rays can’t generate the Bremsstrahlung), and • we are using BOTH characteristic x-rays of the sealed tube target (e.g., Cr, Cu, Mo, Rh) AND continuum x-rays to generate the characteristic x-rays of the atoms in the sample. XRF has been a bulk analytical tool (grind up 50-100 grams of your rock or sample to analyze), though recently people are developing “micro XRF” to focus the beam on a ~100 mm spot. XRF spectrometer (including marketed new) An XRF spectrometer is very similar to an electron microprobe: just replace the electron gun with an xray tube located very close to the specimen; both the characteristic and the continuum x-rays cause (secondary) fluorescence of the specimen, and the resulting x-rays are focused using collimators in either WDS (crystal + counter) or EDS (solid state detector) mode . Detector Principles A detector is composed of a non-conducting or semi-conducting material between two charged electrodes. X-ray radiation ionizes the detector material causing it to become conductive, momentarily. The newly freed electrons are accelerated toward the detector anode to produce an output pulse. In ionized semiconductor produces electronhole pairs, the number of pairs produced is proportional to the X-ray photon energy n where : n E e E e = number of electron-hole pairs produced = X-ray photon energy = 3.8ev for Si at LN2 temper atures Si(Li) Detector Window FET Super-Cooled Cryostat Si(Li) crystal Pre-Amplifier Dewar filled with LN2 Cooling: LN2 or Peltier Window: Beryllium or Polymer Counts Rates: 3,000 – 50,000 cps Resolution: 120-170 eV at Mn K-alpha Spectral Comparison - Au Si(Li) Detector Si PIN Diode Detector 10 vs. 14 Karat 10 vs. 14 Karat Concentration Quantitative Analysis Thursday, April 09, 2015