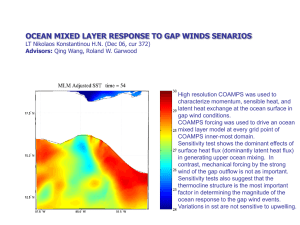
Density
advertisement

Lecture 4b (Ch. 5 of text)
Properties of Seawater (Part II)
Density and Pressure
Why is the deep ocean cold?
Vertical Structure of Temperature
Thermocline
Thermocline is a permanent
hydrographic feature of
temperate and tropical oceans.
Seasonal evolution
of thermocline at
the mid-latitudes
Growing period
Downward heat
transport from
Sep. to Jan.
Decaying period
Vertical Structure of Temperature
Outstanding question:
what sets the depth of the thermocline?
Transfer of Heat to the Ocean (heat flux)
Absorption of solar radiation
decreases rapidly with depth
What controls the ocean’s salinity?
Salinity variations are determined by the
addition or removal of H2O from seawater
Processes such as evaporation and sea ice
formation will increase the salinity
Processes such as rainfall, runoff, and ice
melting will decrease the salinity
Become unchanged with time
halocline
Salinity
How do the water masses move?
c.f. Fig.5.13b
Temperature
Pressure in the Ocean (water is not
absolutely incompressible)
p g h
0
p( z ) g dz
Hydrostatic Equation
z
p ( z )
g
z
Hydrostatic Balance
Seawater density is a function of both
temperature and salinity (so-called TS diagram)
A
B
ρA < ρB
C
ρB < ρC
OCEAN
WATER
MASSES
Vertical profiles
DENSITY: controls the
movement and stability of
the ocean water masses
Density
stratification
Vertical circulation driven by density
Thermohaline Circulation
(18%)
Tropical oceans: pycnocline ≈ thermocline
Mid-latitudes: pycnocline ≈ halocline
High latitudes: no pycnocline formation
Why? (important)
More on the DENSITY
Density: amount of mass per unit volume
Units: kg m-3
Linear Equation for “in situ” Density
T S
Thermal expansion coefficient
Saline contraction coefficient
But water is slightly compressible
Density is actually a non-linear function
of Temperature, Salinity and Pressure !
T S
(T , S , p)
t 1000
-3-3
Kg
m
km m
Taking into account compressibility effects
Potential Temperature
Taking into account compressibility effects
Potential Density
In situ Temperature
Temperature of a particle of water measured at a particular
depth and pressure (no correction for compressibility effects)
Potential Temperature
Temperature that a particle would have if raised adiabatically to
the surface of the ocean (corrects for the effects of compression
occurring at great depth make the particle warmer)
At the ocean surface In Situ and Potential Temperature are the same!
T1
θ1
T1=θ1
Surface
T2
Deep ocean
θ1
T2≠θ1
(T , S , p)
T 1000
( , S , p)
1000
In situ Density
Potential Density
Temperature
Histograms of Temp. and
Salinity in the Oceans
Natural thermostate mechanism
Salinity
tropical cirrus clouds resulting
from deep convection contribute to
long-wave radiative heating of the
tropospheric column, and at the
same time reduce solar insolation at
the sea surface, in this way cooling
the ocean. This dual tropospheric,
long-wave radiative heating and
surface, short-wave radiative
cooling role of cirrus is called the
thermostat mechanism.
The deep convection occurs only
when the SST exceeds 27 C, which
is associated with the so-called
super-greenhouse effect
TS Diagram
Temperature
t 1000 Kg
kmmm-3-3
Salinity
Distribution of T and S in the Ocean
Tracking Water Masses on TS diagrams
AABW: Antarctic Bottom
NADW: North Atlantic Deep
Water
Water
AAIW: Antarctic Intermediate
Water
Tracking Water Masses on TS diagrams
Worlds ocean
Water Masses
Properties of Seawater
Mixing (supplements of Ch.5.6)
How to mix water masses
in the ocean?
Molecular diffusion
Turbulent diffusion
Horizontal Stirring and Mixing
Horizontal Stirring and Mixing
Vertical Stirring and Mixing
Mixing of two water masses with same Density
1
O1T1 S1
2
3
O2T2 S2
Mixing along surfaces of Constant Density
y
+
z
_
Surfaces of
constant density
(i.e. isopycnal)
Mixing along surfaces of Constant Density
y
+
z
_
Surfaces of
constant density
Mixing across surfaces of Constant Density
y
+
z
_
Surfaces of
constant density
Across - Isopycnal diffusive mixing
Definitions of Mixing
y
+
z
b
_
Surfaces of
constant density
Diapycnal Mixing
Definitions of Mixing
y
+
z
b
_
Surfaces of
constant density
Diapycnal Mixing
turbulent diffusion
非絕熱
Diabatic
exchanges with the atmosphere at the surface
T1 S1
Adiabatic
絕熱
T2 S2
changes and
Mixing in ocean interior
Summary of major mixing processes in the Ocean
Surface:
•Wind stirring and vertical
mixing in the surface layer
•Surface fluxes of heat and salt
buoyancy fluxes
•Surface Waves
Interior:
•Along Isopycnal
eddies and fronts
•Across Isopycnal
internal wave breaking
Bottom:
Breaking internal waves over
rough topography
(Important concepts)
Ocean Circulation and Climate
Mixing energy and dissipation of
tides
Mixing rates in the ocean
govern the rate at which
the ocean absorbs heat
and greenhouse gases,
mitigating climate. Global
climate change forecasts
are uncertain in part due
to uncertainty in the
global average ocean
mixing rate. Mixing rates
in the ocean vary
geographically depending
on bottom roughness.
Shown are mixing rates
observed during an
oceanographic survey
across the Brazil Basin in
the South Atlantic Ocean.
Low mixing rates (purple)
were found over the
smooth topography to the
west, and higher mixing
rates (colors) over the
rough topography to the
east (Mauritzen et al.
2002, JGR)
Properties of Seawater
Dissolved Gases (Ch.5.6)
(focus on O2 and CO2)
Air
Total pressure = sum
of partial pressures
Dissolved Gases
(ml l-1)
Seawater
Oxygen
Main regulator is the activity
of organisms (biological
oceanography later)
Dissolved Gases in the Ocean
Oxygen profile
compensation depth
Anoxic environment
Respiration:
Animal, plants and microbial decomposition
Main sources of O2 in the surface layer: photosynthesis
and diffusion across the air-sea interface
Why does the O2-minimum layer coincide with the
pycnocline layer? (important)
Why does the concentration increase with depth toward
the deep seas? (important)
Why is the pH of seawater close to
neutral? (Seawater pH=7.5-8.5)
pH log10[ H ]
pOH ?
Carbon Dioxide and Carbonate system
Why is this important (important)?
1. Regulates temperature of our planet
2. Important for the ocean biota
3. Regulates the acidity of sea water
The pH of water is directly linked to
the CO2 system
Carbon Dioxide and Carbonate system
Sources for acidity in the ocean
Carbonic Acid
H 2O CO2
H
CO
H
HCO
2 3
3
2
HCO3
H
CO
3
Bicarbonate Ion
Carbonate (碳酸鹽)
At the pH of normal seawater,
HCO3- makes up about 80% of
the carbon species
less H+ ions need to be released
More H+ ions need to be released
(b) Photosynthesis and respiration
Why are the
CaCO3 shells
dissolved in the
cold, deep
water, but not in
the warm,
shallow water
(important) ?
pH log10[ H ]
Carbonate Buffer self-regulating system
As temperature is low,
The cold water has a higher
gas-saturation value
As the water becomes
deeper,
The higher pressure also has
a higher gas-saturation value
Thus, the dissolved CO2
amount increases and makes
the water acidic, and melts
the CaCO3 shells that sink to
the deep-sea floor.
→NO Calcareous oozes at
high latitudes
Carbon Dioxide and Carbonate system
Why is it important?
1. Regulates temperature of our planet
2. Important for ocean biota
3. Regulates the pH value of sea water
CO2
Temperature
70 ppm
CO2 changes in the last 300 yr
70 ppm
Industrial Revolution
CO2 changes in the last 50 yr
Oceans
Biosphere
Rock Weathering
How much CO2 can be dissolved by
the ocean (role of ocean uptake in
regulating the global climate)?
Chemical
Process that control CO2
absorption in the ocean
Biological
Physical
Carbon Cycle
Grand Carbon Cycle
The Carbonate System
sources of inorganic carbon
from dissolved CO2 gas
H 2CO2 (aq)
H 2O CO2 ( gas)
2
H HCO3 (aq)
2
H
CO
3 (aq )
from dissolution of Calcium Carbonate
2
2
CaCO3 ( s)
Ca
(
aq
)
CO
3
NOTE:
Biology and Physics participate in the
equilibrium of the carbonate system
Total dissolved inorganic carbon
this is very small
CO
2
not found in this form
CO2 ( gas ) H 2CO2 (aq )
3
2
3
HCO (aq ) CO (aq )
(1)
(2)
formation and decomposition of organic matter
from dissolution of Calcium Carbonate
2
2
CaCO3 ( s)
Ca
(
aq
)
CO
3
Total dissolved
inorganic carbon
Carbon Dioxide and Carbonate system
High pH
+
H 2O CO2 ( gas)
H
CO
H
HCO
2 3
3
2
HCO3
2
H
CO
3
-
Low pH
Distribution of Carbon species in water
+
[ HCO3 ]
[CO32 ]
-
[ HCO3 ]
[CO32 ]
Control of pH
pH log10[ H ]
very rapid reaction in seawater
2
HCO3 (aq)
H
CO
3 (aq )
at equilibrium
[ H ][CO32 ]
K
[ HCO3 ]
hydrogen ion concentration
K
[
HCO
3 ]
[H ]
[CO32 ]
Equilibrium constant
hydrogen ion concentration
+
[ HCO3 ]
[CO32 ]
-
[ H ][CO32 ]
K
[ HCO3 ]
[ HCO3 ]
[CO32 ]
Concept of Alkalinity (鹼度)
H 2O CO2 ( gas)
HCO (aq) H
3
H 2O CO2 ( gas)
CO32 (aq) 2 H
3
2
3
A [ HCO ] 2[CO ]
Alkalinity
A [ HCO3 ] 2[CO32 ]
CO
2
CO2 ( gas ) H 2CO2 (aq )
HCO3 (aq ) CO32 (aq )
[CO2 ] [HCO3 ] [CO32 ]
A [CO2 ] [CO ]
2
3
Why is the pH of seawater close to
neutral?
pH log10[ H ]
seawater
pH=7.5-8.5
So you want the day off :
Lets take a look at what you are asking for :
There are 365 days in the year available for work.
There are 52 weeks in the year, in which you already have 2 days off per week,
leaving 261 (365 − 52x2) days available for work.
Since you spend 16 hours each day away from work, you have used up 170
days (16 x 261 / 24), leaving only 91 days available.
You spend 50 minutes each day in coffee breaks which accounts for 27 days
{[91 x (8 – 50/60)]/24} per year, leaving only 64 (91-27) days available.
With 1 hour lunch period each day, you have used up another 46 days, leaving
only 18 (64-46) days available for work.
You normally spend 2 days per year on sick leave.
This leaves only 16 days available for work.
Normally, we are off for 5 holidays per year, so your available working time is
down to 11 days.
I generously give you 10 days vacation per year, which leaves ONLY 1 DAY
available for work, and I'll be damned if I'm going to let you take that very
day off.