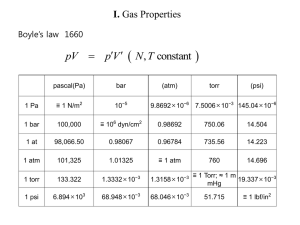
Lecture 1 - University of Oxford Department of Physics
advertisement
Sub-mm/mm astrophysics: How to probe molecular gas Yasuo Fukui Nagoya University Summer School The Gaseous Universe Oxford, 21-23 July 2010 1 Outline Lecture 1 “Sub-mm and mm observations of molecules” Molecular vs. atomic gas/ radiative and collional porcesses / rotational energy levels/ sub-mm transitions/ time scales/ cooling and heating/ chemical processes Lecture 2 “Sub-mm diagnostics; density and temperature” LVG approximation and its application to three high temperature regions N159 and other GMCs in the LMC/ Westerlund 2 super star cluster/ Galactic centre loops Lecture 3 “Giant molecular clouds (GMCs)” GMCs/ star formation/ resolved GMCs in the LMC and M33/ three GMC types/ GMC lifetime/ GMC formation/ HI filaments/ shells or spiral density waves/ difference between disk and center of a galaxy 2 Lecture 1 Sub-mm and mm observations of molecules 3 ISM between stars • We have interstellar medium ISM among stars • ISM consists of gas and dust Mgas/Mdust is 100, Dust grains include most of the heavy elements, abundance ratio; H:He:CNO = 1:10-1:10-4 • Gas consists of neutral and ionized components Here with an emphasis on neutral gas because neutral is dominant, related to star formation and ultimately galactic evolution, Ionized gas is minor in mass and probes UV radiation field, PDR • Neutral consists of molecular and atomic gas – 1951 discovery of 21cm HI – 1970 discovery of 2.6mm CO 4 Molecular vs. atomic • HI gas is less dense, average is 1 cm-3 with a range of 0.01cm-3 to 100 cm-3, temperature average is 100K with a range of 20–3000 K • Molecular gas is dense, average is 1000 cm-3, up to 107 cm-3 or higher • Temperature is low, 10–20 K in the disk, can be higher in high-mass star forming regions • but is higher in the Galactic center, 30–300 K, due to not-well known heating 5 Some chemistry • HI is converted into H2 on grain surface because gas phase reaction is very slow, exception the first stars form without dust grains • H2 is readily dissociated if Av is small, less than ~ 0.2 mag • but can survive if Av is more than 1 mag • Other molecules are often formed via ion neutral reactions • At very high densities more than 107cm-3 molecules freeze onto dust grain surface 6 Radiative transitions The Einstein coefficients • (a) Spontaneous emission: A21n2 • (b) Photo absorption: B12Iνn1 • (c) Stimulated emission: B21Iνn2 (Electric dipole transition) 7 Collisional Excitation Excitation De-Excitation • C coefficient : (N: density, σ: cross section, v: velocity) 8 Excitation temperature • For non-LTE case (more realistic in molecular cloud), we can define the “Excitation temperature” as follows; (*Especially, Tex of the spin excitation (e.g. HI 21cm line) is called “Spin temperature”, Ts) • Brightness temperature 9 Detailed balancing • Ex. Simple two level system rate equation • Collision dominated • Radiation dominated Critical density • When Iν → 0 • Critical Density: • ncrit << n(H2) : • ncrit ~ n(H2) : • ncrit >> n(H2) : LTE excited but subthermal unexcited 12 My recommendation • Keeep physical constants in mind • Ready to make order-of-magnitude estimate Planck const.: h = 6.63×10-27 erg s Boltzmann const.: k = 1.38×10-16 erg deg-1 1 eV = 1.60×10-12 erg(e.g., 1eV~kT => T〜104 K) electron mass: me = 0.911x10-27 g proton mass: mp = 1.67x10-24 g etc. 13 Non-LTE case (Photon trapping) • Photon escape probability, β (0 ≤ β ≤ 1 ) • Effective A coefficient: A21 → A21β • Effective critical density: ncrit = A21/C21 → A21β/C21 • Large Velocity Gradient (LVG) model Molecular cloud (Castor 1970; Goldreich & Kwan 1974) - Spherical Velocity - Slab Tk, n(H2)14 Heating processes • Based on the ionization of ISM components by an energetic radiations. Then, electrons quickly (~1Myr) interact with the ISM and thermalize. – Cosmic rays; heat gas to 10 K (Black 1987; Lequex 2002) – Photoelectric effect on small dust grains and PAH (Watson 1972; de Jong 1977; Draine 1978; Bakes & Tielens 1994) – Ionization of atoms and molecules (e.g., HCO+) froze-in condition is good approx. MHD – X-ray – Chemistry – Mechanical heating 15 Cooling processes Cooling by line radiation processes is dominant. Proportional to n2. • Atomic gas – Forbidden lines. (< few 1000 K, e.g., CII) – Lyman α (> few 1000 K) • Molecular gas Cooling by the line radiation from molecules – CO, H2O and other molecules 16 Thermal equilibrium curve WNM CNM Unstable Wolfire et al. 95 criterion for instability: P 0 L 0 (Field et al. 69, Wolfire et al.1795) Heating and Cooling processes for the thermal equilibrium curve (NW~1019 cm-2) Atomic gas (solid lines: cooling, broken lines: heating) Wolfire et al. 199518 Tk = 40 K Molecular cooling • CO is the dominant cooling line for low n and T • H2O and other molecules are dominant for n > 106 cm-3 and T > 200 K Goldsmith & Langer 1978 19 Temperature and density balance of atomic gas (Goldsmith et al. 2007) 20 Temperature • • • • • kinetic temperature (Maxwell distribution), Tk excitation temperature, Tex radiation temperature, Planck law, Trad color temperature, Tc dust temperature, Td These temperatures are generally not the same Collisional coupling between dust and molecules at density higher than 104 cm-3, Tk equal to Td 21 Excitation 1 Upper state • • • • Lower state Electronic state (1-104 eV) Vibration (10-2-10-1 eV) Rotation (10-3-10-2 eV) Spin (~10-6 eV) 104K ~ 1eV 22 Excitation 2 • Hydrogen molecules are not observable in radio. Too high energy levels. Only in absorption. • Carbon monoxide CO and others can be observed rotational energy levels, high excitation vibration. cf. electronic, spin-spin interaction • Sub-mm transitions generally higher excited states ratio between J and J’ gives density/tempearture. 23 Molecular cloud 24 Goldsmith 1987 Time scale 1 • Crossing time scale – Velocity width (5-10 km/s): dv – Molecular cloud size (1-100 pc): r = 105 – 107 yr 25 Time scale 2 • Free fall time a:initial radius, ρ(0) : initial density n : initial number density, n = n(H) + 2n(H2) 26 Time scale 3 • Cooling time shorter than its dynamical time isothermal is a good approximation 27 Time scale 4 How frequently do molecules meet? • C(s-1) = n (cm-3) s (cm2) V(cm/s) n(density) ~ 103 cm-3 s(cross section) ~ πa2 ~ 10-16 cm2 V(velocity) ~ 105 cm s-1 [mV2 ~ kT] t ~ 1/C ~ 108 [s] ~ 1 [yr] 28 Time scale 5 • Formation time scale of H2 (Hollenbach & Salpeter 1970; Jura 1974) γ:sticking probability for incident H atoms. <v2>: mean thermal velocity of H atoms. <σg>: average grain cross section. n1, n2 & ng: number density of HI, H2 and grains, respectively [yr] 29 Time scale 6 30 Koyama & Inutsuka 2000 Formation of H2 in gas phase • Permitted processes in warmer regions H+ + H → H2+ + hν H2+ + H → H2 + H+ e- + H → H- + hν H- + H → H2 + e- In very dense regions(> 108 cm-3), 3 body reaction 3H → H2 + H 2H + H2 → 2H2 This process is important in the early Universe. Very dense & hot HI cloud → molecular cloud 31