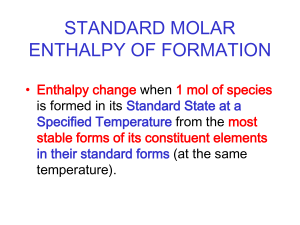
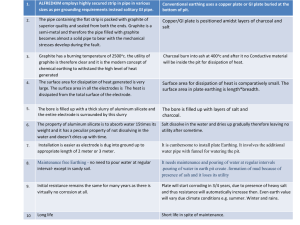
Recent Graphite Research at the Nuclear Science and Engineering
advertisement

Sudarshan K. Loyalka Nuclear Science and Engineering Institute Particulate Systems Research Center University of Missouri, Columbia, USA September 16, 2014 Tier 1: MELC Integrated Code H d We G ORConsolidated Timeline of Nuclear Tier 2: Mechanistic CodesSCDAP, CONTAIN, VICTORIA Codes Safety Technology Phenomenological Experiments(PBF, ACRR, FLHT, HI/VI, HEVA) Evolution Phebus FP, VERCORSEuropean Codes Deterministic Bounding Analysis Probabilistic Risk Informed Analysis Chicago Critical Pile Atomic Energy Act of 1946 (AEC) Risk Informed Regulation Atomic Energy Act of 1954 USS NautilusShippingport TMI-2 AEC 9-11-2001 Chernobyl NUREG-1150 MOX LTA revised1465 1940 1950 1960 1970 Windscale 1980 NRC 1990 NPP Siting Study 2000 2010 NUREG 1465 alternatesourceterm TID 14844 NUREG 0772 source term Nuclear Technology Outlook NP-2010 and Gen-IV WASH 1400 Optimistic Guarded Pessimistic Emerging Issues MOX, High Burnup, Life Exension Environmental ConcernsGlobal Warming and Where are we going ? Vulnerability to Terrorism P ( M W th ) R ( m iles ) 17 thus for, P =3000 R 13.2 m iles 1/ 2 , Need to understand and predict: FP diffusion through the particles and graphite FP release into and plateout from the coolant Moisture and dust interactions Renewed interest in graphite-fueled reactors Need for measurement of modern nuclear graphite properties and interactions Research areas: ▪ ▪ ▪ ▪ ▪ ▪ ▪ ▪ ▪ ▪ HTGR source term issues Graphite dust particle generation Graphite oxidation Adsorption of fission products on graphite Fission products diffusion in graphite Fission products transport to aerosols Dust adhesion to surfaces Dust re-suspension Coagulation Emissivity Graphite dust is produced during PBR operation Sources of generation Fuel handling system Pebble on pebble abrasion Pebble on reactor components Experimental setup Surface area and Sliding Distance Surface properties of graphite samples * Data from nitrogen adsorption at 77 K. The BET surface area is calculated using the Brunauer - Emmett - Taylor equation. The total pore volume is measured at maximum nitrogen pressure. R. Troy, R. Tompson, T. Ghosh, and S. Loyalka, "Generation of graphite particles by rotational/spinning abrasion and their characterization," Nuclear Technology, vol. 178, (2012) 241-257 . A Paper on sliding friction is to appear in Nuclear Technology (2014-15). Others on nuclear graphites in preparation. Objectives To predict the oxidation rate of nuclear-grade and matrixgrade graphite under various air ingress accident conditions for VHTR Study the oxidation attack mechanism Characterize the surface and microstructural changes Model the oxidation rate in air using the Arrhenius equation In the chemically-controlled Regime I of graphite oxidation, reaction rate pre-exponential factor apparent activation energy reaction velocity constant reaction order Ea , A and n are determined experimentally. The slope of the mass loss plot = - Ea/R, where R is the ideal gas constant. From collision theory, before a reaction can occur the molecules of reactants must have an energy of activation Ea above their normal, or average energy. There is a strong correlation between density of nuclear graphite and its physical and mechanical properties. 2.50 Density (g/cm3) 2.00 1.77 g/cm3 IG-110 bulk density 1.85 g/cm3 NBG-18 bulk density 1.50 1.00 0.50 NBG-18 0.00 0.00 Surface of rod 1.00 2.00 3.00 4.00 Distance from surface (mm) rod orientation IG-110 5.00 6.00 7.00 Pure NBG-18 Oxidized NBG-18 Pure IG-110 Oxidized IG-110 IG-110 oxidized more rapidly and more uniformly in the same experimental conditions as NBG-18 IG-110 is more porous and therefore experiences larger increases in surface area in the kinetic regime ORNL manufactured GKrS by using the German A3 recipe but with modern materials and hot pressing method (we thank ORNL for providing us with this material). “A3 recipe” 64 wt% natural graphite 20 wt% resin binder 16 wt% petroleum coke graphite While nuclear-grade graphite is almost fully graphitized at temperatures around 2800°C, matrix-grade graphite is only “partially graphitized” <2000°C fuel fabrication temperature Air ingress into matrix graphite can affect retention properties of the fuel and we have shown the oxidation rate is high in the kinetic regime 0.6 Oxidation rate (g/hr/g) 0.5 0.4 0.3 0.2 0.1 0 800 1000 1200 IG-110 1400 Temperature (K) NBG-18 1600 GKrS 1800 2000 Jo Jo Lee, Tushar K. Ghosh, Sudarshan K. Loyalka, “Oxidation rate of graphitic matrix material GKrS in the kinetic regime for VHTR air ingress accident scenarios,” Journal of Nuclear Materials, 451 (2014) 48-54. Jo Jo Lee, Tushar K. Ghosh, Sudarshan K. Loyalka, “Oxidation rate of nuclear-grade graphite IG-110 in the kinetic regime for VHTR air ingress accident scenarios,” Journal of Nuclear Materials, 446 (2014) 38-48. Jo Jo Lee, Tushar K. Ghosh, Sudarshan K. Loyalka, “Oxidation rate of nuclear-grade graphite NBG-18 in the kinetic regime for VHTR air ingress accident scenarios,” Journal of Nuclear Materials, 438 (2013) 77-87. Silver Carbon Gold Palladium J Nanopart Res (2011) 13:6591–6601 6599 Fig. 8 SEM images of some larger nanoparticles a1 gold, b1 silver, and c1 palladium. Energy dispersive X -ray spectra (EDS) of the particles a2 gold, b2 silver, and c2 palladium, confirms the particles for gold, silver, and palladium, respectively 1 3 Inlet: Argon gas with particles at higher temperature Outlet Small notch to position TEM copper grid Aluminum rod in ice cold water Simulated experiment of Romay et. al. – NaCl particles in dry air Case 1: 100 nm particles Case 2: 482 nm particles 96.5 cm mesh with 2,066,400 volume cells mesh with 7,029,360 volume cells Cross-sections of two different meshes used in this computation Boundary conditions Inlet flow velocity 4.42 m/s Tube wall temperature 293 K Totally four meshes (465,520, 899,160, 2,066,400, 7,029,360 volumes) were used in this study. Objectives Review pervious works on the adsorption of iodine to graphite. Examine experimental methods used in the past. Determine the usability of data with adsorption isotherm equations and Polanyi's Potential. Design and build experiments for iodine adsorption with more accurate means for generating and measuring iodine vapor Obtain adsorption isotherms of IG-110: For a single particle (up to 300 °C) For bulk powder (up to 1000 °C) Model data with for newly acquired data: isotherm models, kinetics, … Review of iodine literature published in Progress of Nuclear Energy (Volume 73, May 2013, Pages 2150) Summary of the graphites reviewed in the paper: Some data for high temperature adsorption on graphite from the review. 1073 K Isotherm 1273 K Isotherm Obtain adsorption isotherms of IG-110: Both single particle and bulk powder forms Temperature range: Low (room to 200 °C) and high (300 to 1000 °C) Electrodynamic balance (EDB) for single particle adsorption experiments. Develop models from newly acquired data: Isotherm Models Polanyi Potential Adsorption Kinetics (if possible) Packed bed-tube furnace experiment for bulk adsorption measurements. Objectives Characterize physical properties of the nuclear grade graphite (i.e. density and porosity). Determine the diffusion coefficient of silver through nuclear grade graphite. Model the diffusion of silver through nuclear grade graphite. Russia USA/FRG Offermann Present Work 1 Present Work 2 Q (kJ/mol) 193 154 164 D0 (m2/s) 5.3x10-4 5.3x10-9 1.0x10-8 D (800oC) 2.14x10-13 1.69x10-16 1.04x10-16 D (1150oC) 4.37x10-11 9.57x10-15 1.18x10-14 1.03x10-15 1.67x10-15 C x 35.8023 erfc 17415.6 x C x 6.8171 erfc 11781.0 x Samples were annealed for 4 days at 1150o C. D (1600oC) 2.20x10-9 2.69x10-13 2.67x10-13 Thomas R. Boyle, et al., "Measurement of Silver Diffusion in VHTR Graphitic Materials." Nuc .Tech. 183(2) (2013) 149-159. Problem: R. Troy, R. Tompson, T. Ghosh, and S. Loyalka, "Generation of graphite particles by rotational/spinning abrasion and their characterization," Nuclear Technology, vol. 178, pp. 241-257, 2012. . VHTR aerosols are not nicely shaped for computations Jagged shapes Agglomerations Porous Materials Z. Smith and S. Loyalka, "Numerical Solutions of the Poisson Equation: Condensation/Evaporation on Arbitrarily Shaped Aerosols," NUCLEAR SCIENCE AND ENGINEERING, vol. 176, pp. 154-166, 2014. R. Troy, R. Tompson, T. Ghosh, and S. Loyalka, "Generation of graphite particles by rotational/spinning abrasion and their characterization," Nuclear Technology, vol. 178, pp. 241-257, 2012. Objective: To understand the adhesion of graphite particles and fission products (with and without the influence of surface roughness) to reactor materials of interest Hastelloy X, Haynes 230, and Alloy 617 ▪ Oxidation Important for reactor design, safety and system analysis because it increases surface roughness which affects emissivity and decay heat removal Plays important role on sustainability of structural integrity of materials over long period. ▪ Adhesion roughness may affect adhesion of particles to surfaces due to reduced contact area Adhesion force is critical in understanding re-suspension of particles under LOCA Hastelloy X material surface conditions: Oxidized for 5, 10, and 15 min @ ~800 0C and 10 -6 torr As receive and polished surfaces Mica as a benchmark (standard) Particle of interaction: Graphite cluster as a particle (size ~ 6 µm ) produced in VHTR among fission products aerosols Conditions and parameters of interest: Environment – Air; Approach rate -1.7848 µm/s; Measurements of Adhesion Force (nN) and work of energy (mJ/m2) obtained when a 6 μm diameter Irregular Graphite Particle Probe (Approximated as a Sphere) Interacts with Graphite Sprinkled Hastelloy X Surfaces of Different Conditions. Approach-Retract Rate is 1.7848 μms−1. Substrate Location 1 Location 2 Location 3 Fadhesion W Fadhesion W Fadhesion W (nN) (mJ/m2) (nN) (mJ/m2) (nN) (mJ/m2) HX polished 18.90 1.34 24.23 1.71 46.09 3.26 HX as received 14.64 1.04 13.57 0.96 27.97 1.98 HX 5min Ox 15.70 1.11 11.44 0.81 12.50 0.88 HX 10min Ox 10.90 0.77 11.97 0.85 10.90 0.77 HX 15min Ox 18.90 1.34 17.84 1.26 18.37 1.30 Adhesion Force (nN) and work of energy (mJ/m2) calculated using the JKR Theory and Assuming a Spherical Graphite Particle witha 6 μm Diameter Estimated using Optical Microscope. Substrate Graphite (C) Highly Ordered Pyrolytic Graphite Fadhesion W Fadhesion W (nN) (mJ/m2) (nN) (mJ/m2) MICA 6849.30 484.49 12718.70 899.66 Hastelloy X 2637.30 186.55 4897.30 346.41 HOPG/Graphite 820.00 58.00 2827.40 199.99 The adhesion force was relatively small in all cases, especially, when when compared to the theoretical values. Graphite particle was a cluster and not well characterize and surface asperities of the particle where not included. Large difference between calculated values from JKR theory and measured values may be due to assuming the particle to be spherical in shape. The pikes seen during measurement may be caused by many factors large loading force applied on sample by the probe. Interaction of particle with graphite first then with Hastelloy X or other nano- graphite particles on the surface. Mokgalapa, N. M., Ghosh, T. K., & Loyalka, S. K, “Graphite Particle Adhesion to Hastelloy X: Measurements of the Adhesive Force with an Atomic Force Microscope,” Nuc.Tech., 186(1) (2014) 45-59. VHTRs generate charged aerosols during normal operation. All reactors can release charged aerosols during severe accidents. Charged aerosol behavior is complex. Charge effects on coagulation Electrostatic forces Current codes and models are inadequate, relying on numerical techniques which do not account for charge effects. p ,q i, j p ,q p ,q . p ,q i, j p ,q Kernel p ,q e p ,q Repulsion 1 p ,q 1 e Attraction p ,q pqe 2 2 0 kT d p d q n 1 d p Q s max ne d 1 , n e , Z p n 0 d p , n e Q a * p n e 1 Sampling probe Spark generator Transfer function Unknown distribution ~ Z 1 p 2 1 2 ~ ~ ~ Z p 1 Z p 1 Z p 1 2 2 2 ~ 1 Z p , , , Grad Students/Post Docs F. De-La-Torre Aguillar Sunita Boddu Matthew A. Boraas Tom Boyle Sean Branney Shawn Campbell Sergio Correra Andrew Gordon Rajesh Gutti Paul Harden Jo Jo Lee Leroy Lee Ray Maynard Ryan Meyer Naphtali Mokgalapa Shawn Nelson Giang Nam Nguyen John Palsmeier Michael Reinig Matthew Simones John-David Seelig Zeb Smith Lynn Tipton Raymond Troy Kyle Walton Nathan White Jason Wilson Tushar Ghosh – Professor & Director of Graduate Studies Sudarshan Loyalka - Curators’ Professor Fellow: ANS, APS; PE Mark Prelas - Professor & Director of Research Fellow: ANS; PE Robert Tompson - Professor Dabir Viswanath - Emeritus Professor & Chair of ChE Fellow: AIChE; PE NERI-C , VHTR Consortium, NSEI lead (with NCSU and MST) , 2007-2013, NERIC-08-043 Infrastructure for FP/Aerosol Transport, 2010Computations for Aerosol and FP transport, 2011-2014, NEUP -964 Adsorption/Diffusion of FP in Graphite, 20112015, NEUP-2982 Measurements and Modeling of Emissivity (2014-2017), NEUP-6282 Graduate Fellowships (NRC), GAANN (DOEd)