超导磁体 - 国家同步辐射实验室
advertisement

Cryogenic System
WANG Li
Shanghai Institute of Applied Physics, CAS, China
The Eighth OCPA Accelerator School
July 27-Aug. 6, 2014, Xiuning, Anhui
2014-08-03
1
Contents
Introduction to cryogenic engineering
How to reach the low temperature
How to design a cryogenic system
Homework
References
2
Introduction to Cryogenic
Engineering
3
Introduction to Cryogenic Engineering
"Cryogenics the science and art of
producing cold"
Cryogenic Engineering
The design and development of systems and components which
produce, maintain, or utilize low temperatures.
Field of Cryogenics
Involving temperatures below 123K (-150oC) because the normal
boiling points of the so-called permanent gases, such as helium,
hydrogen, neon, nitrogen, oxygen, and air, lie below 123K.
根据在不同的低温温度区域、获得低温的方法及研究对象,又可把制
冷技术分为普冷技术和深冷技术。习惯上把普冷技术称为制冷技术,
把深冷技术称为低温技术。
4
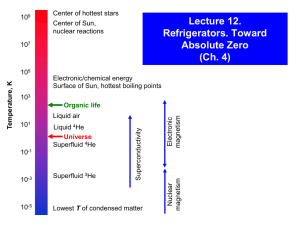
Temperature Scale (I)
10,000 K
Surface of sun
制冷
1,000 K
Water freezes
100 K
Cryogenics
Liquid oxygen (90.18K) Begins (123K)
Liquid argon (87.28K)
Air liquefies (78.8K)
Liquid nitrogen (77.36K)
Liquid neon (27.09K)
Helium
Liquid hydrogen (20.27K)
cycle
Liquid helium4 (4.21K)
refrigerator
Liquid helium3 (3.19K)
低温
10 K
1K
0.1 K
Magnetic
refrigerator
0.01 K
Dilution
refrigerator
0.001 K
极低温
5
104
102
100
10-2
Superfluid 3 He
10-4
10-6
Coldest temperature (bulk sample)
10-8
Bose-Einstein and Fermionic condensates
10-10
High T
ULT
Cryogenics
Temperature (K)
106
Plasma
108
Supernova explosions
Center of hottest stars
Fusion (hydrogen) bomb
Center of sun
Fission (atomic) bomb
Surface of hottest star
Surface of Sun
Room temperature
Metal boiling points
Organic life
Warmest superconductor (140 K)
Air liquefies
4He liquefies/Superfluid 4He
Space background (3 K)
Nuclear
reactions
1010
Conventional
refrigeration
Temperature Scale (II)
Coldest atomic temperature (sodium atoms)
Coldest nuclear temperature (rhodium nuclei)
Tempscale4 .cdr
6
Chronology of Cryogenic Engineering
~ 1850
1850
1877
1879
1879
1883
1892
1898
1895~1905
1908
1911
1907~1922
1926
1933
1934
1939
1940s
1942
1946
1946
1948
1952
Pre-cryogenics Era of Natural Ice
Artificial production of ice and the first air-cycle
refrigerator
Oxygen Liquefied
First frozen meat cargo from Australia to London
Domestic ammonia refrigerators
Liquefied nitrogen and oxygen
Dewar developed silvered vacuum vessel for
cryogen storage
Liquefied hydrogen
Industrial air liquefaction
Onnes liquefied helium
Discovered superconductivity
Cryogenic industries in the U.S.
Cryogenically propelled rockets
Magnetic cooling
First turbine expansion engine
First liquefied natural gas (LNG) plant
Ductile-brittle transformation
The V-2 weapon system was test-fired
Collins’ helium liquefier
Cryobiology (Discovery of cryoprotectants)
First 140 ton/day oxygen system built in
America.
Cryogenic Engineering Laboratory of U.S.
National Bureau of Standards (NBS)
1957
1958
1959
1959
1960
BCS theory of superconductivity
Multilayer cryogenic insulation (MLI)
72 in Liquid Hydrogen Bubble Chamber
Large NASA liquid-hydrogen plant at
Torrance, California, completed
Large-scale liquid-hydrogen plant completed
at West Palm Beach, Florida.
LNG tankers
1961
Type 2 Superconductors
1961
Space rocketry
1963
60 ton/day liquid-hydrogen plant completed
by Linde Co. at Sacramento, California
1966
Dilution refrigerator using He3-He4 mixture
1969
3250-hp dc superconducting motor
constructed for ship drive application
1970
Liquid oxygen plants with capacities
between 60,000 m3/h and 70,000 m3/h
developed
1971
Cryogenic wind tunnel
1983
Magnetic resonance imaging
1986
Ceramic superconductors
1960
2000 ~~~
7
Benefits of Low Temperatures
Cryogenics required for many quantum effects
– Superconductivity 超导(无电阻、完全抗磁性)
– Superfluids 超流(液氦II表现出几乎为0的流动阻力)
– Bose-Einstein condensates 玻色-爱因斯坦凝聚(原子在接近0K时特
殊物态)
Cryogenics is an enabling technology
– High magnetic fields (MRI, NMR, motors, accelerators)
– High precision and high speed measurements
– Low noise electronics and electromagnetics
– Liquefied fuels for high density
– Cryopreservation
– Cryosurgery
– Cryogrinding
– ……
8
Applications Involving Cryogenic Engineering
Cryobiology
Superconducting
Magnets & SRF
cavities
Space
Propulsion
MAGLEV
Trains
Cryosurgery
Elect. Power
Transmission
Air
Products
LNG & LH2
Automobiles
Liquefied
Natural
Gas
Steel
Manufacturing
LHe
Cryopumping
for
High Vacuum
Nuclear and
Particle
Physics
Cryogenic
Wind-tunnels
LH2
Cryomedical
MRI
Superconductivity
Research
Food
Processing
LOX
LAr
LN2
Material
Recycling
Cryocoolers
for Electronics
& SC device
CRYOGENICS
LNG
Elect.
Motors
9
Early Nobel Prizes
and Discoveries in Physics
Year
Red = Cryogenic phenomena
1900
Max Planck (1900)
Albert Einstein (1905)
Nobel Prizes
(begun in 1901)
Onnes (1908 Liquid helium)
1910
Onnes (1911 Superconductivity)
(1913) Properties of matter at low T
and production of liquid helium
Kamerlingh Onnes
(1918) Energy quantization
1920
(1921) Particle nature of light
Bose & Einstein (1924 condensate)
Louis de Broglie (1924)
Willem Keesom (1926 solid He-4)
Werner Heisenberg (1927)
S. Bose Einstein
(1929) Wave nature of particles
1930
William Giauque (1933)
P. Kapitza (1938 Superfluid He-4)
1940
Nobel Prize, 1978
(1932) Uncertainty principle
(1949) Thermochemistry and
Adiabatic demagnetization
10
Year
Recent Nobel Prizes in Physics
1970
1972
1973
Red = Cryogenic phenomena
Bardeen, Cooper, and Schrieffer
Esaki, Giaever, and Josephson
Theory of superconductivity
Tunneling in semi- & superconductors
1978
1980
Kapitza, et al.
Inventions & discoveries in low temp. physics
1985
1987
Klitzing
Quantized Hall effect
Bednorz and Muller
High-temperature supercond uctors
De Gennes
Ordering in matter (superco nd. & superfluids)
Lee, Osheroff, and Richardson
Ch u, Tannoudji, and Ph illips
Laughlin, Stormer, and Tsui
Superfluidity in He-3
Laser cooling and trapping of atoms
Fractional quantum Hall effect
Co rnell, Ketterl, and Wieman
Bose-Einstein condensation in dilute gases
Abrikosov, Ginzburg, and Leggett
Theory of superconductors a nd superfluids
Lauterbur and Mansfield
Magnetic reso nance imagin g (MRI)
1990
1996
NIST 1997
1998
2000
NIST 2001
2003
Medicine 2003
11
Cryogenic Applications and Operating Regions
10 6
低温超导
REFRIGERATION POWER (W)
Air liquefaction
小型制冷机
Accelerators
& Fusion
10
(Commercial)
10
10
10
10
Turbo-Brayton
1 GJ
Mid-size
(Special)
Cryopumps
SMES
GM+JT
FCL
Maglev
Magnetic
LTS
Electronics
He 5
JT
NbN
Elect.
10
GiffordMcMahon
FCL
Motors
Bearings
Wireless
1MJ
Micro-SMES
uency
q
e
r
f
Low
MRI
10
H2O cryotraps
Transformers
10
10
Mixed-gas JT
Stirling
Transmission
lines
Turbo-Brayton/Claude
1 TJ
Largesize
LNG
H2 ZBO
TE
IR
HTS
SQUIDs
H2
JT
50
TEMPERATURE (K)
Space
radiators
100
N2
JT
300
12
低温技术在粒子物理领域的应用
• 近十余年来,人类对物质结构的研究进入了大科学工程甚至超级
科学工程的时代,已建、在建并拟建大批的超导的加速器、对撞
机、探测器、核聚变装置、同步辐射光源和自由电子激光装置等
等。依靠这些超导科学研究装置,人类对物质结构的探索正期待
着重大突破。
• 低温技术( 2K-4K氦低温冷却技术)与超导技术(各类超导磁体、
超导射频腔等)的大规模应用是这些大科学工程的基本特征。现
代低温与超导技术随着这些大科学工程的研制迅速发展起来。
• 超导加速技术是实验物理技术,极其复杂的超导磁体和超导射频
腔,以及低温恒温器装置与以氦制冷机为核心的低温系统是其中
的关键设备。
13
欧洲CERN核子中心-强子对撞机(LHC)
•
1250个场强为8.3T的主二极(偏转)磁体,磁场强度梯度为223T/m的主四极(聚焦)磁体400个,分布
在周长为26.7km的加速器环上。
•
整个环等分为8个区域,每一区域内各个单元由一台制冷功率为18kW/4.5K的氦制冷机通过低温
传输线来冷却。
•
为达到8.3T场强,选用较为成熟的Nb-Ti合金作为超导线材后,基于NbTi合金特性,工作温度选
定为1.9 K,冷却介质是1.8-1.9K的超流氦(He II)。
ITER:国际热核聚变实验堆
• 超导托克马克装置
• 装置中心是高温氘氚等离子体环:15MA的等离子体电
流,核聚变反应功率达50万kW,每秒释放多达1000个
高能中子。
• 18个大型超导环向场线圈,将产生5.3T的环向强磁场。
线圈的作用是产生等离子体电流和控制等离子体位形。
• 系统罩于一个低温杜瓦中,坐落于底座上,构成实验堆
本体。
• ITER纵场导体和中心螺管导体采用Nb3Sn,其余导体采
用NbTi材料,超导磁体总重量约10130吨。
• 所有磁体采用4.5K超临界氦迫流冷却,低温制冷系统提
供55kW制冷量,80K的热辐射屏需要的冷量大约是
660kW。
• 大型氦低温技术和系统是保障ITER装置可靠工作的前提
和基础,用以支持其内部各种大中型超导磁体的运行。
15
未来的加速器及对撞机ILC:高能量和高亮度
•
直线对撞机(International Linear Collider-ILC):正负电子对撞机。采用低温超导加
速技术,由两台大型超导直线加速器组成,除了超导磁体,主加速器是一台由8000多个
9-cell超导加速腔组成的总长为 12km 的庞大机器,加速梯度高达31.5 MV/m, 可以把电
子束加速到250GeV的超高能量并进行对撞,质心系能量达到 500GeV,以后还可扩展到
1TeV。需建造约12个总冷量45kW/2K的超流氦制冷系统维持其运行。基本建设和加速器
设备的造价估算为67亿美元,需要2000名科研和工程技术人员持续工作5年以上。
16
高能轻子加速器:中微子工厂和介子对撞机
•
中微子工厂和介子对撞机 (Neutrino Factory & Muon Collider-NFMC):高能轻子加速
器。由于μ子的质量约比电子大200 倍,同步辐射损失小,容易在环形加速器中被加速到
更高能量。 NFMC是 ILC的竞争对手,更可能是它以后的高能加速器,两者具有互补性。
正在英国RAL建设的MICE实验装置,用于实验验证NFMCC中的关键技术之一-离子化冷
却μ介子技术,μ介子冷却通道采用超导螺线管磁体(18个)和液氢吸收器,以及低温
(液氢、液氦)冷却技术。
3.5 MW
Proton
Source
Hg-Jet Target
Decay
Channel
Buncher
Acceleration
Helical
Cooler
Pre Accel
-erator
Ring
Cooler
Collider
~ 4 km
Bunch
Merger
Li Lens
Cooler
17
美国能源部二十年大科学工程发展规划 (I)
美国能源部2003年11月公布的二
十年中长期大科学工程发展规划中
共有28项,拟投资数十亿美元。这
些大工程项目中的80%是以低温与
超导技术为工程基础。
“这些大科学工程将使科学发生革
命,使美国科学位于世界前沿,将
会产生重大科学发现,对人类社会
做出重大贡献”
-Spencer Abraham
(美国前能源部长)
18
美国能源部二十年大科学工程发展规划 (II)
19
19
国际拟建大科学装置中的低温与超导技术
• 未来的国际正负电子线性直线加速器ILC计划采用近万个9壳1.3GHz纯铌超导
腔,需建造12个总冷量45kW/2K的超流氦制冷系统维持其运行;
• 美国散裂中子源装置SNS拟采用81个805MHz的纯铌超导腔和总冷量
2.4kW/2.1K的制冷系统;
• 欧洲CERN拟建的高强度质子直线加速器SPL将采用178个704MHz的纯铌超导
腔和总冷量4.5kW/2K的制冷系统;
• 德国GSI实验室在建的研究反质子和离子的实验装置FAIR全部采用超导磁体,
需要总冷量30-40kW/4.4K的制冷系统;
• 德国DESY实验室拟建的欧洲X ray-FEL装置将采用928个1.3GHz纯铌超导腔,
仅直线加速部分即需要2.45kW/2K的超流氦制冷系统;
• 美国康奈尔大学建造的能量回收型加速器ERL需要390个7壳1.3GHz纯铌超导
腔和约10 kW/2K的制冷系统;
• 英国建在德拉斯珀瑞的G4LS-ERL/FEL装置需要102个1.3GHz纯铌超导腔和约
3.5kW/1.8K的超流氦制冷系统。
• FRIB, LCLSII, ERL, ……
20
中国的国家级大科学工程装置
SRF-B
SRF-A
上海第三代同步辐射光源装置
BESIII
SCQ-A
SCQ-B
北京正负电子对撞机
重大改造工程
21
北京正负电子对撞机重大改造工程
BEPCII低温设备
新增三类超导设备:
一对撞区聚焦磁体(SCQ)
一对高频加速腔(SRF)
一个探测器磁体(SSM)
一套氦低温系统:
2套500W/4.5K氦制冷机系
统
三种冷却方式
(哈工大设计制图)
22
22
SSRF超导设备和低温系统
正在运行:
• 3套500MHz Single-cell超
导射频腔,液氦浸泡式冷却
• 一套氦低温系统:
650W/4.5K氦制冷机系统
拟建:
• 1套超导3次谐波腔+1套
SCW,液氦浸泡式冷却
• 一套氦低温系统:
650W/4.5K+100W/2.0 K
氦制冷机系统
23
加速器和光源装置中的超导设备及低温技术
冷却对象
功能
超导射频腔(SC
Resonance
加速电子
Frequency CavitySRF cavity)
低温永磁波荡器
(Cryogenic
Permanent Magnet
Undulator-CPMU)
超导插入件(SC
Undulator-SCU, SC
Wiggler-SCW)
产生同步辐
射光
磁体材料
工作温区
冷量范围
冷却方式
冷却技术或冷源
纯铌腔体
4.5K or
1.8K~2K
~几百瓦
液氦浸泡式
大型氦制冷技术
Liquefier+LHe fill-in
永磁体
NdFeB、
PrFeB
50K-150K
~几百瓦
对流+热传
导
LN2 or GN2对流冷却,或者
小型低温制冷机传导冷却
超导材料
NbTi/Cu、
Nb3Sn/Cu
超导磁体(四极铁、
超导材料
聚焦、偏转
二级铁、弯铁、六
NbTi/Cu、
等
极铁等)
Nb3Sn/Cu
4.5K
2K~4.5K
几瓦~几十
瓦(取决
于动态负
载量级)
大型氦制冷技术
液氦浸泡式; Liquefier+LHe fill-in or
对流+热传 小型低温制冷机技术
导;热传导 Cryocooler+LHe fill-in
by thermo-syphon or by
GHe-recondensening;
Cryocooler by conduction
液氦浸泡式;
对流+热传 大型氦制冷技术 or 小型低
几瓦~百瓦
导等
温制冷机技术
24
气体储罐、纯化系统等
液氦杜瓦、低温阀箱、低温传输管线、过冷
换热器、气液分离器、测量控制系统等
How to reach the LT?
低温恒温器等
25
How to reach the
low temperature?
26
Approach to obtain the low temperature
Approach to obtain the low temperature (>1K)
节流; 绝热膨胀; 绝热放气制冷;减压降温;相变制冷(液体气化制冷、
固体升华制冷);He3-He4稀释制冷;绝热退磁制冷;涡流制冷;热电制冷;
吸附制冷 ……
Refrigeration and Liquefaction cycle
Two adiabatic expansion processes:
Isenthalpic expansion Throttle valve
Isentropic expansion Turbine and piston engines
Cycles: Linde-Hampson, Brayton,Claude, Kapitza, Heylandt, Collins, etc.
Cryocoolers or Cryocoolers+JT
G-M coolers (Simon expansion)
Pulse tube coolers
Stirling coolers
……
27
Refrigeration and Liquefaction Cycle
Simple refrigerator
Simple liquefier
• To reject heat at high temperature
• To reject heat at high temperature
• To absorb heat at low temperature
• To produce liquid at low temperature
Power
Power
P > 0
T
Heat
Load (TL)
P > 0
T
P1
Gas
supply
Q
Q
Q
Cold sink
(TH)
P < 0
T
P < 0
T
Liquid
vessel
P1
P2
dT=0
T1
T
dP=0
T2
ds=0
P2
Power
Power
P2
Cold
sink (TH)
P1
dT=0
T
ds=0
dP=0
T’2
28
s
s
Refrigeration vs Liquefaction Cycle
Refrigeration
Liquefaction
Closed loop system
Open loop system
• Operate as a closed loop, constantly
circulating the same working fluid.
• Liquid product is removed at cold end
and equivalent the make-up gas added at
warm end.
• No accumulation or withdrawal of fluid in a
refrigerator.
Balanced refrigerant flow
Unbalanced refrigerant flow
• Mass flow rate of the refrigerant is the
same at all points of the cycle.
• Mass flow rate of return stream is
smaller than that of stream being cooled
by just the amount of liquid removed.
Absorb heat at low temperature
Refrigerant production
• Fluid coolant evaporates constantly at low
temperature end and returns to complete
cycle.
• Cold liquid is produced but the
countercurrent refrigerant effect of the
product withdrawn has been lost.
Joule-Thomson isenthalpic expansion
The J-T process is isenthalpic,
dh 0
The isenthalpic, or J-T, expansion coefficient
JT
T
P h
For a perfect gas,
JT
1
cP
v
T T v
P
Pv RT
R
v
v
P T
T P
Conclusions
h
•
A perfect gas would not experience any
temperature change upon expansion
through an expansion valve.
•
The more imperfect the gas, the better.
•
A liquid is the most imperfect gas as
known.
•
To achieve the liquid state before the JT expansion.
•
Among liquids, the most non-ideal ones
are the highly polar fluids
•
Ammonia, solfur dioxide, and
fluorocarbons (R series refrigerants)
Therefore, for a perfect gas
JT
1
cP
v
T T v 0
P2, T2, h
P1, T1, h
dh 0
30
The J-T Cycle
Refrigerator
Liquefier
31
Conclusions
•
•
The inversion curves
Maximum inversion temperature below ambient temperature: Neon, hydrogen,
helium.
For these gases, a system can not use the Joule-Thomson effect alone in
producing low temperatures, and other methods, such as expansion engines or
precooling, must be used.
Fig. Isenthalpic expansion of a real gas
32
Isentropic expansion
For an isentropic expansion,
ds 0
The isentropic expansion coefficient,
T T v
S
P S cP T P
P1, T1,
h1, s
S
S 0
if the volumetric coefficient of expansion,
W
dP 0, dT 0
v
0
T P
P2, T2,
h2, s
This is the case for all fluid (Water between 0oC~4oC is one
exception).
For an ideal gas,
Pv RT
v / T P R / P v / T
S v / cP
33
The Brayton Cycle
Refrigerator
Liquefier
34
h
Compare
h
1
h S
cP
and
and
S
S
v
1
T
v
T P
cP
v
v 1
1 v
v
v T
T
T
T P c P T P
T P c P
v
RT
h S
cP
PcP
h S
•
•
•
•
•
when
v0
cP
At low temperature and high pressure, v 0 , so, use J-T expansion at low T and high P.
Near critical point all fluids, c , which is exactly the condition desired for J-T
P
expansion.
Therefore, when the expansion is for producing liquid or occurs near or below the critical
point, J-T expansion may be indistinguishable thermodynamically from isentropic
expansion.
Real expansion engine have difficulty operating in two-phase region, may be only about
85% of isentropic.
Hardware reasons to prefer valves to engines.
35
Examples
36
Modified Claude system with LN2 precooling
T1
T2
ORS
Recycle
compr.
GHe
buffer
GHe
LHe
LN2
Drier
LHe storage
dewar
Air
T1
T2
ORS
Recycle
compr.
GHe
buffer
GHe
LN2
LHe
Drier
Air
LHe storage
dewar
Qc
Qc
37
System performance parameters
COPreal
FOMR
COPideal
卡诺比/卡诺效率/制冷机效率:循
环热力过程完善度
Figure of Merit or Percent Carnot
QTC
heat _ absorbed@TC
COP
compressio
n _ work
Wcomp
y
m f
m comp
Wcomp
制冷系数:制冷循环热效率
Coefficient of Performance
m f
Wcomp
mass_ liquefied
mass_ compressed
液化率
m comp
compression _ work
mass_ liquefied
compression _ work
mass_ compressed
38
Thermodynamically ideal systems
Ideal refrigeration system with isothermal-sources:Carnot refrigerator
TH
TH
2
1
QH
T
Wnet
TC
3
QC
4
QC
C-R
TC
s
12: Isothermal reversible compression, reject QH;
23: Isentropic (adiabatic, reversible) expansion, THTC;
34: Isothermal reversible expansion, absorb QC;
41: Isentropic compression, TCTH .
The COP of Carnot refrigeration
system is independent of the
refrigerant and is limited by TH and
TC only.
Q
W
Q
C
H
net 0
COPideal
TC s
Q
Q
m
TC
C
C
Q
TH s m
TC s TH TC
m
W net
Q
H
C
39
,For
300
TH = 300K, COPideal
3
1 10
100
10
1
C OP
i
0.1
4
3.0110
0.01
3
1 10
10 4
1
5 5
3.32210
1 10
0
0.01
100
T
200
i
300
300
5
1 10
4
1 10
3
1 10
1
100
C OP
i
10
1
0.1
3.33310
3
0.01
3
1 10
0
0.01
100
T
200
i
300
300
40
,
Ideal liquefaction system with isentropic expansion
Analysis: CV: {COMP+EXPAN+LD}
m
f
m
y 1
P2
P1
dT=0
T
W
m
f hf m
hH m
f (hf hH )
Q
H
net
Wideal
Q H
(h f hH )
(h f hH ) TH (s1 s2 ) (h f hH ) TH (s f sH ) 0
f
f
m
m
ds=0
dP=0
s
41
Examples
42
气体储罐、纯化系统等
液氦杜瓦、低温阀箱、低温传输管线、过冷
换热器、气液分离器、测量控制系统等
低温恒温器等
How to design a cryogenic system?
43
How to design a
cryogenic system?
44
How to design a cryogenic system?
Tasks and components of cryogenic system
低温系统的主要功能及主要组成
Cooling Cycles 冷却循环方式
Overall design 总体设计
Cooling capacity vs heat load/leakage 制冷量和热负载(漏热)确定
Cooling schemes 冷却方式选择
Operation modes 运行工况(或模式)优化
Machine components 主要设备的确定
Cryogen properties and Material properties
低温工质物性和材料低温性质
2K Cryo-system
45
He
Gas
Tank
A Typical Cryogenic System
Including:
Compressors
Cold Box
HEXs
Thermodynamics/Heat-transfer/Fluid-mechanics/Fluid-Material-properties/Numerical-simulation
Project Budget: Heat load vs. Refrigeration (Static & dynamic heat loads)
Cooling sources: Working-medium vs. Refrigerator or Cryocooler vs. Cryogens
Cooling method: Bath vs. 2Φ vs. Supercritical vs. Subcooled vs. LHe II
Refrigerator plant: Compressors/Heat-exchangers/Expanders/J-T valve/Liquid-dewars/cold-comp. /Absorbers/etc.
Equipment: Gas-tanks/Purifiers/Filters/Transfer-lines/Valves/Pumps/Instrument-air/cooling-water/etc.
Instruments: Temperature/Pressure/Flow-rate/Liquid-level/Fluid-quality/etc.
Cryo-operation: Capital bill for Auto-control vs. Labor & materials costs vs. Down-time-loss/etc.
Vacuum chamber@ 300K
Turbin expanders
Free-Molecular
Heat Transfer
JT valves
Heat Flux
Vacuum
Cryogen Dewar
Cooling Tube
Radiation
LN2
Cryo-Valve Box
Cold Mass @ 4.5K
2Φ Helium
ΔP, ΔT, Δρ, Δχ,
M*Cp*ΔT
Convection
MLI
Conduction
ΔP, ΔT, Δρ, Δχ,
Cooling of Permanent current leads:
LN2 Shield @ 80K •Conduction-cooled
Vacuum
Pump
MLI
Conduction
CRYOSTAT
•Ghe-cooled
Permanent current leads:
•Cu leads
•Binary leads: Cu leads + HTS leads
46
Basic Knowledge
Thermodynamics 工程热力学
• Refrigeration/Cooling capacity Cold mass thermal capacity
Heat taken away from the cold mass, i.e. cooling down
• Refrigeration static heat load
Heat transferred from outside worlds, i.e. keeping cold
• Refrigeration dynamic heat load
Heat added to target, i.e. beam-based heating, RF heating, AC losses, etc.
•
•
•
•
Heat transfer 传热学
Thermal calculations for vacuum-insulation;
Thermal conduction in solid supports and links in mechanical systems;
Heat convection between gas, liquid, and solid in cooling systems.
Boiling and condensing
Fluid mechanics 流体力学
• Single- and two-phase fluid flow calculations;
• Safety device sizing and verifications.
Material properties 制冷工质和材料低温物性
• Cryogen properties;
• Mechanical material properties.
Numerical simulation 有限元或有限差分数值模拟
• Commercial computational software, i.e. Fluent, Ansys, Aspen Plus……
47
Tasks and Components of Cryogenic System (I)
• Production of refrigeration power or cryogen -冷源:提供冷量或者低温工质
Refrigerator/liquefier (recycle compressor, ORS, cold box) or cryocoolers– 制冷机或液化机、小型制
冷机(GM, PTR, or cryocooler + JT, etc.)等
• Distribution of cryogen – 低温工质的贮存、分配和输送
V-L separator & cryogenic valve boxes & transfer-lines –气液分离器、低温阀箱和传输管线等
• Cooling of components – 热负载的冷却
Cooling circuit (sub-cooler, cold pump, recondenser, cooling pipes, etc.) –冷却回路(过冷器,冷泵,
再冷凝器等); Cold mass (SC device, test samples) –冷质量;Cryostat (including supports,
thermal insulation, thermal shields, vacuum vessel, current leads, quench protection, etc.) – 低温恒
温器
• Process control and monitoring – 低温系统的监控
Cryogenic instrumentation and control system – 低温监控系统(温度、压力、流量、液位、电压等测控
元件,PLC模块等)
48
Tasks and Components of Cryogenic System (II)
• Variability and flexibility of refrigeration to handle different modes of
operation – 不同运行模式下冷量的调节
• Cryogen recovery and storage as liquid or gas – 低温工质的回收和储存
Cryogen recovery and storage systems (gas collection headers, dewar, tank, recovery
compressor)- 杜瓦、气罐、回收压机等
• Cryogen purification - 低温工质的纯化
Cryogen purification system (filters, absorbers, driers, gas analyzers, internal or external
purifiers) – 过滤器、吸附器、干燥器、气体分析仪、内/外纯化器等
• Handling of safety aspects – 低温安全
Safety device (pressure relieve valves, burst-disc, Oxygen monitor, etc.)
• Accommodation to cryogenic system – 低温系统的布局
• Others (instrument air system, cooling-water system, vacuum system, etc. )- 仪表
空气系统、冷却水系统、真空系统等
49
Cooling Cycles
Cooling Cycle to be Chosen:Closed- or Open-cycle
冷却循环方式:开式或闭式
Refrigeration loads 制冷量
Operating temperature range 运行温度范围
Operating period 运行时间
Convenience of operation 操作简便性
Cost 成本(capital cost & operation cost 设备和运行)
Stability and reliability etc. 运行可靠性、稳定性等
50
Overall Design
Study refrigeration loads (冷量的确定)
Choose cooling principle/scheme (冷却方式的选取)
Operating modes(运行工况/模式)
Choose machine components (主要设备的确定)
51
冷却对象(冷质量):超导设备及工作温度
超导加速器设备类型
超导磁体
通过大电流产生强磁场,实现高能粒子的聚焦、偏转、旋转、摇摆、散射。
超导二极、四极、六极、超导螺旋管、超导弯铁、超导插入件(Wiggler,
Undulator)、超导异型磁体等等.
超导射频腔
通过高频功率源加载产生交变强电场,加速带电粒子。
单壳、多壳,低频至高频(MHz-GHz).
功率输入器
电流引线、功率耦合器或波导管等。
超导体的工作温度条件
低温超导磁体和超导射频腔:1.8K(超流氦)-4.5K
电流引线: 4.2K-300K,高温超导电流引线:30K-77K
功率耦合器或波导管:1.8K-300K
52
Cooling capacity vs Heat-load 制冷量和热负载(漏热)
― How big the
refrigerator/liquefier
/cryocooler has to be?
制冷系统的规模
300K 真空容器
辐
射
漏
热
功
率
输
入
器
漏
热
Static heat 静态漏热
•Radiation heat 辐射漏热
•Conduction heat 导热漏热
•Free-molecular heat
transfer 剩余气体传热
动
态
漏
热
支
撑
导
热
漏
热
各
种
管
线
漏
热
测
量
引
线
漏
热
低
温
阀
门
漏
热
中间温区冷屏(液氮冷屏)
辐
射
漏
热
功
率
输
入
器
漏
热
支
撑
导
热
漏
热
各
种
管
线
漏
热
测
量
引
线
漏
热
液氦温区冷质量
LN2
低
温
阀
门
漏
热
超
导
线
接
头
漏
热
LHe
53
超导腔和超导插入件低温冷却系统的热负载
•
低温系统的热负载主要包括:超导腔热负载、超导插入件热负载和超导设备与制冷机之
间低温工质的传输与控制设备的热负载三部分。
•
超导设备的热负载又分为静态热负载和动态热负载;传输设备的热负载含低温传输管线、
各类阀箱、制冷剂储存杜瓦等设备的热负载。
•
超导设备静态热负载是指在超导体低温恒温器中的低温设备与室温之间由于热辐射、热
传导等而产生的固定热量传递。通常,会在超导设备恒温器中采取多种措施以将低温下
热负载减小到最低程度,如在液氦容器与室温间通过中间温度冷屏(如液氮冷屏、20K
冷屏)及高真空进行热隔离,在低温超导设备与室温束流管道间设置波纹管及中间温度
热隔离段等。
•
超导高频腔的动态热负载一部份来源于高频功率在超导腔壁的残留电阻上产生的热损耗,
另一部份来源于高频功率在耦合器上产生的热损耗。其中前一部份与超导腔的性能和运
行参数直接相关。
•
超导插入件的动态热负载则主要来源于束流辐射等。
54
Cooling principle/scheme 冷却方式的选取
Mainly to be determined by 主要取决于:
•Temperature range of operation 运行温度要求 (温度/压力稳定性、温度均匀性等)
•Cooling efficiency 冷却效率
•Geometry of cold mass 冷质量的形状及结构
•Heat loads (static and dynamic) 漏热
•Cryogen flow conditions (single or two-phase) 低温工质流体状态(单相或两相流)
•Heat exchange and heat transport conditions (precooling, recooling, …) 热交换或传热状况
•Allowed pressure levels (magnets, cavities, etc.) 允许的工质压力范围-压力容器及管道要求
•Spatial distribution of the cold components (locations of magnets, cavities, coldboxes, cryostats,
dewars). 冷组件的空间分布及空间可利用率
Mainly Including 主要包括:
•
Bath cooling or Pool boiling
浸泡式冷却
•
Forced flow cooling
迫流冷却
•
Conductive cooling
热传导冷却
•
Thermal-siphon cooling
热虹吸冷却
55
Bath cooling or Pool boiling
Direct cooling using liquid cryogen storage or closed-cycle refrigerator. The cold
mass is immersed in the cryogen pool (saturated, subcooled, superfluid)
•
Most efficient cooling mode good
•
Solid and Leak-tight cryogen vessel to contain the cold mass technical
difficult for large size or complex geometry
•
Electrical insulation and mechanical strength hard
•
High vapor pressure when quenched safety issue
•
Long time recovery after quench cost
Liquid Helium I
Superfluid Helium II
56
Liquid Helium I
Saturated He I at about atmospheric pressure (1.013-1.25 bara) with a temperature of
4.2~4.5 K is used for bath cooling. Heat flux of 20 J/g/s of helium flow is absorbed by
the latent heat of helium. The helium is boiling at constant temperature, defined by the
equilibrium vapor pressure in the cooling loop.
Lhe consumption rate: 1 L/hr ( 0.033 g/s)=0.7 W Heat load
Cooling power: 1 W=1.4~1.5 L/hr (0.05 g/s)
Superfluid Helium II
Saturated He II at about sub-atmospheric pressure (16-31 mbar) with a temperature of
1.8~2.0 K is used for bath cooling. Heat flux of 22~23 J/g/s of helium flow is absorbed
by the latent heat of helium II. The heat flux is conduction-transferred at 0.6~0.7
W/cm^2 in helium II at constant temperature, defined by the equilibrium vapor
pressure in the cooling loop.
Lhe consumption rate: 1 L/hr ( 0.041 g/s)=0. 91 W Heat load
Cooling power: 1 W= 1.1 L/hr (0.0451g/s)
57
Forced flow cooling
Indirect cooling. Cryogen flows through metal tubes that attached onto
the cold mass.
•
Very efficient cooling mode good
•
Simple structure good
•
Cooling of the SC conductor joints need special care
•
Electrical insulation complicated
•
Cryogen flow control sophisticated
•
Low vapor pressure when quenched less safety issue
•
Quick recovery after quench
BEPCII SCQ magnet
58
He cooling loop
N2 cooling loop
Two-phase Helium
Two-phase helium is used as the forced flow in piping that is attached onto the cold
mass. Same as the saturated liquid, the helium flow at 1.0 g/s may absorb 20 J heat flux
by its latent heat. The helium boils at constant temperature, and its fluid quality
increases along the cooling path. The temperature is defined by the equilibrium vapor
pressure in the cooling loop that is normally established by the suction pressure of the
helium compressor. High heat transfer rate may be obtained at small temperature
difference up to 1 W/cm^2 in the nucleate boiling region.
Lhe consumption rate: 1L/hr=0.7 W Heat load
Cooling power: 1 W=1.4~1.5 L/hr
Subcooled single-phase Helium
Below critical pressure of 2.3 bar, heat is absorbed by the sensible heat of the helium with
a corresponding increase in temperature, 0.7 J/(g/s-K) at T=0.1K. This is a factor of 30
less than in the two-phase cooling at the same helium mass flow rate. Higher mass flow
rates lower the temperature rise. Normally, the maximum allowed temperature rise is
about 0.1 to 0.2 K and limits maximum length of component to be cooled in series.
Because in subcooled helium, vapor bubbles can not occur, the flow instabilities such as
59
in two-phase flow conditions are excluded.
Supercritical Helium
Above critical pressure of 2.3 bar, heat is absorbed by the sensible heat of the helium with
a corresponding increase in temperature, 0.5 J/(g/s-K) at T=0.1K. Higher mass flow rates
lower the temperature rise. Normally, the maximum allowed temperature rise is about 0.1
to 0.2 K and limits maximum length of component to be cooled in series. Because in
supercritical helium, vapor bubbles can not occur, the flow instabilities such as in twophase flow conditions are excluded.
Conductive cooling
Indirect cooling based on heat conduction. Completed dry cooling.
•
Simplest in fluid flow and pressure control good
•
Cooling capability and efficiency big concerns
•
Small stability margin only for low loss magnets operating in a pure steadystate regime
T
Qc (T ) A
L
60
小型低温制冷机冷却系统
Conduction-cooled
Thermosyphon-cooled
61
小型低温制冷机的选型
目前,常用的小型制冷机主要有GM型制冷机和脉冲管(Pulse Tube)型制冷机,其
性能比较如下表所示。
脉管制冷机(PT-415)
G-M制冷机(RDK-415D or KDE415)
单机最大冷量范围
40W﹫45K,1.5W﹫4.2K
35W﹫50K,1.5W﹫4.2K
维护周期
低温部件无需 维护,其余部件周期
25000小时
整机需要维护,周期10000小时
运行方位
垂直安装运行,最大偏离角为10o
可任意方位安装,冷量有损,最大15%损
失量
磁场影响
低温蓄冷器处磁场小于1.5T,其它低
温部件对磁场无要求,也不会影响磁
场分布,旋转阀电机处磁场应小于
0.04T
低温蓄冷器处磁场小于1.5T,低温部件处
磁场小于0.5T,旋转阀电机处磁场应小于
0.04T
振动
在冷头轴向方向有4-7μm量级的振动,
在冷头横向方向有2μm量级的振动;
采取减振措施,冷头轴向方向振动可
至<<1μm。
在冷头轴向方向有20μm-30μm-量级的振
动,在冷头横向方向有10μm量级的振动。
采取减振措施,冷头轴向方向振动可至
1~5μm量级。
厂商
Cryomech, USA
日本住友,或南京柯德
62
Cryomech/USA coolers
Valve Motor
Ballast Tank
Rotary Valve
1st Stage Cold Head
2nd Stage Cold Head
Cooler Top Plate
Æø¿â
ÀäÈ´Æ÷
Àä¶Ë»»ÈÈÆ÷
Èȶ˻»ÈÈÆ÷
ѹËõ»ú
x
С¿×·§
ÐîÀäÆ÷
1.5W/4.2K PTR-415 PTR cryocooler Produced by Cryomech/USA
Âö³å¹Ü
63
Sumimoto/Japan coolers
1
2
7
8
1.5W/4.2K SRDK-415D GM cryocooler
Produced by Sumimoto/Japan
3
4
5
6
64
Conduction-cooled SCU
Cryostat for SC magnet with indirect cooling
Conduction-cooled current leads
Horizontal bath cryostat for a wiggler magnet
65
Thermo-syphon cooling
Gravity (density difference)-driven flow.
SC magnet He fill/vent turret
LHe vessel
Cryocoolers 4K/60K
Current lead
assemblies
LHe piping
1
He
fill
pipe
2
20 K radiation shield
LHe vessel
HTS leads
He
recondenser
60 K radiation shield
Cryostat vacuum vessel
Cold mass support
Beam chamber
Beam chamber
thermal link to
cryocooler
20K radiation shield
60K radiation shield
Beam chamber
@ 20K
LHe
Heater
RF fingers
SC coils
Cryocoolers 20K/60K
3
4
66
Operation modes 运行工况/模式
Cool down
Normal operation
Warm up
Failure modes
•
Quench
•
Vacuum failure
•
Electricity failure
•
Cryogen leakage
•
……
67
Cryogen Properties 低温工质物性
常用低温工质的基本性质
氧 O2
氦 4He
氦 3He
(n)–正常氢
(e)–平衡氢
氮 N2
空气
32.00
4.003
3.016
2.016
28.018
28.966
K
90.188
4.224
3.191
20.39(n)
20.28(e)
77.36
78.9/81.7
Tm
K
54.4
—
—
13.96
63.2
—
临界温度
Tcr
K
154.78
5.2014
3.324
33.24(n)
32.90(e)
126.26
132.55
临界压力
Pcr
MPa
5.107
0.2275
0.1165
1.297(n)
1.287(e)
3.398
3.769
三相点温度
Ttr
K
54.361
—
—
13.95(n)
13.81(e)
63.15
—
三相点压力
Ptr
kPa
0.152
—
—
7.2006(n)
7.0406(e)
12.536
—
饱和液体密度
ρL
kg/m3
1142
125
60
≈70.8
808
≈873
饱和蒸气密度
ρV
kg/m3
4.8
≈15.5
≈22
1.34
4.61
4.48
密度(标准状态)
ρ0
kg/m3
1.4289
0.1785
0.1345
0.0899
1.2506
1.2928
气化热(1atm)
rV
kJ/kg
212.76
20.8
8.5
447
199
205.5
熔化热(近似)
rm
kJ/kg
13.95
5.7
—
58.7
25.8
项 目
符号
分子量
M
沸点
Tb
熔点(近似)
单位
68 —
Properties of He
T [K]
P=3 atm
P=100 atm
P=2 atm
I: L-V two-phase
P=1 atm
IV
V
II: Near critical point
P=0.5 atm
III: Subcooled LHe
II
5.2 K
IV: Supercritical
III
I
V: GHe
S [kJ/kg-K]
c =69.6 [kg/m3]
Critical point
Tc =5.201 [K] Pc =2.275 [bar]
Normal boil point
Lower lambda point
Tnbp =4.224 [K] Pnbp =1.013 [bar] nbp_L =124.96 [kg/m3] nbp_V =16.89
[kg/m3]
Tλ_Lower =2.177 [K]
Pλ_Lower =50.36 [mbar] λ_liq =146.21 [kg/m3]
Upper lambda point
Tλ_upper =1.763 [K]
Pλ_upper =30.13 [bar] λ_liq =180.39 [kg/m3]
69
4He相图
Critical
point
Tc =5.201 [K]
Pc =2.275 [bar]
Normal
boil point
Tnbp =4.224 [K]
Pnbp =1.013 [bar]
Lower
lambda
point
Tλ_Lower =2.177 [K]
Pλ_Lower =50.36
[mbar]
Upper
lambda
point
Tλ_upper =1.763
[K] Pλ_upper
=30.13 [bar]
70
N2相图
71
Change in volume from liquid at normal
boiling point to gas at 300 K and 1 atm
72
Material Properties 材料低温性能
Some suitable materials for cryogenic use include:
• Austenitic stainless steels e.g. :304, 316L, 304L, 321
• Aluminum alloys e.g. :6061, 6063, 5083, 1100
•Copper e.g. :OFHC, ETP and phosphorous deoxidized, 磷青铜、铍青铜等,
TU2/TP2/T2
•Brass (H62/H65)
•Fiber reinforced plastics: G –10, s2-glass, carbon fiberglass, etc.
•Niobium & Titanium (frequently used in superconducting RF systems)
•Invar (Ni /Fe alloy) useful in making washers due to its lower coefficient of
expansion
•Indium (used as an O ring material)
•Kapton and Mylar (used in Multilayer Insulation and as electrical insulation)
• Quartz (used in optical windows)
•……
73
Thermal Expansion Coefficient
α=1/L (δL/δT)
•Large amounts of contraction can occur when materials are cooled to cryogenic
temperatures.
•α goes to 0 at 0 slope as T approaches 0 K
•α is T independent at higher temperatures
•For practical work the integral thermal contraction is more useful
Points to consider:
•Impact on alignment
•Development of interferences or gaps due to dissimilar materials
•Increased strain and possible failure
•Impact on wiring
•Most contraction occurs above 77 K
Thermal Stress:Secondary stress
Th
E (T ) L dT
Tc
74
75
Integral Thermal Contraction
Roughly speaking:
•Metals – 0.5% or less
•Polymers – 1.5 – 3%
•Some amorphous materials have 0 or even negative
thermal contraction
76
Thermal Conductivity
77
2 K Cryo-system
How to make 2K Super-fluid Helium ? 减压降温
Saturated Vapor Pressure of Helium
latent heat of vaporization
1.2 bar
4.4 K
0.03 bar
2.0 K
Temperature [K]
Enthalpy [ J / g ]
Pressure [bar]
Gas State
20 J / g
Liquid State
2.0 K
Temperature [K]
Liq. He 1 l
1 / 8 kg
1 / 8 x 20 = 2.5 kJ
Liq. He 1 l / hr -- 2.5 / 3.6 = 0.7 W
Use the latent heat of vaporization of helium
Cooling Power
Cold Pump
Large System CEBAF, LHC, ….
Warm Pump
Small System KEK
1W -- Liq. He Consumption 1.4 l /hr
78
Pumping
(Warm
or
Cold Pump)
JT1
Normal Helium
T~ 4.2 K
JT - Heat
Exchanger
4.2 K
2.2 K
JT2
89 %
62 %
Super-fluid
T~ 1.8 K
79
Cooling schemes
Refrigerators operating at 2K or lower need to pump on a liquid helium bath to
lower the saturation vapor pressure below 31 mbar. The pumping (helium
compression to atmospheric pressure) can be done at warm or at cryogenic
temperatures or in a mixed mode. Due to additional pressure drop in the helium lines
and the heat exchangers, a pressure ratio of nearly 40 to reach atmospheric pressure
is needed.
When planning refrigeration at sub-atmospheric pressure, one has to decide,
whether pure warm compression, or pure cold compression, or partly cold with
subsequent warm compression shall be applied. Accordingly, several types of
thermal cycles are generally considered to produce refrigeration below 2 K, the “purewarm” compression cycle based on multiple vacuum pumps, the “pure-cold”
compression cycle based on multistage cold compressors (generally 4~5 stages), and
the “mixed” compression cycle based on a combination of cold compressors in series
with warm sub-atmospheric compressors.
For the layout of the helium system, capital costs, operating expenses, capacity of
available pumping facilities must be taken into considerations. The most important
criteria for the choice is the cooling power required at sub-atmospheric
pressure.
80
Cold Box
4.4K LHe I
Cold Box
4.4K LHe I
81
Cold Box
4.4K LHe I
Cold Box
4.4K LHe I
82
Homework
83
Determine the ideal-work requirement for the liquefaction of
nitrogen, beginning at 101.3 kPa and 300K.
From the T-s diagram of nitrogen:
hH = 462 [J/g] at 101.3 kPa and 300K
hf = 29 [J/g] at 101.3 kPa and 77K
sH = 4.42 [J/g-K] at 101.3 kPa and 300K
sf = 0.42 [J/g] at 101.3 kPa and 77K
84
References
85
86
87
88