X-ray Diffraction: Principles and Practice
Ashish Garg and Nilesh Gurao
Department of Materials Science and Engineering
Indian Institute of Technology Kanpur
Layout of the Lecture
Materials Characterization
Basics
Diffraction
X-ray Diffraction
Crystal Structure and X-ray Diffraction
Different Methods
Importance of X-ray Diffraction
Phase Analysis
Texture Analysis
Stress Analysis
Particles Size Analysis
………..
Summary
Materials Characterization
Essentially to evaluate the structure and
properties
Structural Characterization
Diffraction
X-ray and Electron Diffraction
Microscopy
Spectroscopy
Property Evaluation
Mechanical
Electrical
Anything else
Time Line
1665: Diffraction effects observed by Italian
mathematician Francesco Maria Grimaldi
1868: X-rays Discovered by German Scientist
Röntgen
1912: Discovery of X-ray Diffraction by
Crystals: von Laue
1912: Bragg’s Discovery
Electromagnetic Spectrum
Generation of X-rays
Commercial X-ray Tube
X-ray Spectrum from an Iron target
Short Wavelength Limit SW L ( nm )
Continuous spectrum
I CS AiZV
Characteristic X-ray Moseley’s Law
C (Z )
I K Bi (V V k )
λSWL
n
m
12400
V
Use of Filter
Ni filter for Cu Target
Crystal Systems and Bravais Lattices
Structure of Common Materials
Metals
Copper: FCC
-Iron: BCC
Zinc: HCP
Silver: FCC
Aluminium: FCC
Ceramics
SiC: Diamond Cubic
Al2O3: Hexagonal
MgO: NaCl type
Diffraction
A diffracted beam may be defined as a beam
composed of a large number of scattered rays
mutually reinforcing each other
Scattering
Interaction with a single particle
Diffraction
Interaction with a crystal
Scattering Modes
Random arrangement of atoms in space gives rise
to scattering in all directions: weak effect and
intensities add
By atoms arranged periodically in space
In a few specific directions satisfying Bragg’s law: strong
intensities of the scattered beam :Diffraction
No scattering along directions not satisfying Bragg’s law
Diffraction of light through an aperture
d
1
0.9
0.8
0.7
0.6
Intensity
0.5
0.4
0.3
0.2
0.1
0
-15
-10
-5
0
5
10
15
1
0.9
0.8
0.7
0.6
Intensity
0.5
0.4
0.3
0.2
0.1
0
-15
-10
-5
0
Minima
sin θ
λ
10
15
Maxima
sin
n
a
5
n = 0, 1,..
2n 1
2a
n = 1, 2,..
Young’s Double slit experiment
d sinθ = mλ, m = 1,2,3…..
Constructive Interference
d sinθ = (m+½)λ, m = 1,2,3…..
Destructive Interference
Interference
Phase Difference = 0˚
Phase Difference = 180˚
Phase Difference = 90˚
Interference and Diffraction
Bragg’s Law
n=2d.sin
n: Order of reflection
d: Plane spacing
a
=
h k l
: Bragg Angle
2
2
in
out
2
2
Path difference must be integral multiples of the wavelength
in=out
Braggs Law
d
n
2 sin
1
sin
d
2
Geometry of Bragg’s law
The incident beam, the normal to the reflection plane,
and the diffracted beam are always co-planar.
The angle between the diffracted beam and the
transmitted beam is always 2 (usually measured).
Sin cannot be more than unity; this requires
nλ < 2d, for n=1, λ < 2d
λ should be less than twice the d spacing we want to study
Order of reflection
Rewrite Bragg’s law λ=2 sin d/n
A reflection of any order as a first order reflection from planes,
real or fictitious, spaced at a distance 1/n of the previous spacing
Set d’ = d/n
λ=2d’ sin
An nth order reflection from (hkl) planes of spacing d may be
considered as a first order reflection from the (nh nk nl) plane of
spacing d’ = d/n
*The term reflection is only notional due to symmetry between incoming and
outgoing beam w.r.t. plane normal, otherwise we are only talking of diffraction.
Reciprocal lattice vectors
Used to describe Fourier analysis of electron concentration
of the diffracted pattern.
Every crystal has associated with it a crystal lattice and a
reciprocal lattice.
A diffraction pattern of a crystal is the map of reciprocal
lattice of the crystal.
Real space
Reciprocal space
Crystal Lattice
Reciprocal Lattice
Crystal structure
Diffraction pattern
Unit cell content
y
x
y’
x’
Structure factor
y’
x’
Reciprocal space
a*
b*
c*
bc
a (b c )
Reciprocal lattice of FCC is BCC
and vice versa
ca
a (b c )
ab
a (b c )
001
a
010
b
c
100
Ewald sphere
1 k'
hkl
1
2
d hkl
2 hkl
k
Ewald sphere
Limiting sphere
Ewald sphere
J. Krawit, Introduction to Diffraction in Materials Science and Engineering, Wiley New York 2001
Two Circle Diffractometer
For polycrystalline Materials
Four Circle Diffractometer
For single crystals
2 Circle diffratometer 2 and
3 and 4 circle diffractometer 2θ, ω, φ, χ
6 circle diffractometer θ, φ, χ and δ, γ, µ
www.serc.carleton.edu/
Hong et al., Nuclear Instruments and Methods in Physics Research A 572 (2007) 942
NaCl crystals in a tube facing X-ray beam
Powder Diffractometer
(400)
(410)
(220)
(330)(221)
(310)
(311)
(222)
(320)
(321)
(211)
(210)
(200)
(111)
(110)
(100)
Calculated Patterns for a Cubic Crystal
Structure Factor
N
Fhkl
fne
2 i ( hu n kv n lw n )
Intensity of the diffracted beam |F|2
1
−
−
−
−
h,k,l : indices of the diffraction plane under consideration
u,v,w : co-ordinates of the atoms in the lattice
N : number of atoms
fn : scattering factor of a particular type of atom
Bravais Lattice
Reflections possibly present Reflections necessarily absent
Simple
All
None
Body Centered (h+k+l): Even
(h+k+l): Odd
Face Centered
h, k, and l: mixed
h, k, and l unmixed i.e. all
odd or all even
Systematic Absences
Permitted Reflections
Simple Cubic
BCC
(100), (110), (111), (200), (210), (211),
(220), (300), (221) ………
(110), (200), (211), (220), (310), (222)….
FCC
(111), (200), (220), (311)…..
Diffraction Methods
Method
Wavelength
Angle
Laue
Variable
Fixed
Rotating
Crystal
Powder
Fixed
Fixed
Specimen
Single
Crystal
Variable (in Single
part)
Crystal
Variable
Powder
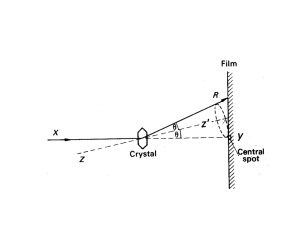
Laue Method
Transmission
Zone axis
Reflection
Zone axis
crystal
crystal
Film
Incident beam
•
•
•
Incident beam
Film
Uses Single crystal
Uses White Radiation
Used for determining crystal orientation and quality
Rotating Crystal Method
Determination of unknown crystal structures
Powder Method
Sample
Incident Beam
Film
• Useful for determining lattice parameters with high precision and for
identification of phases
Indexing a powder pattern
Bragg’s Law
n = 2d sin
For cubic crystals
a
d hkl 2
2
2
h k l
w hich gives rise to
2
sin θ
θ
S1
h k l
2
(for front reflections) or
2W
S2
θ 1
(for back reflections)
2
W
2
2
2
4a
2
w hich is a constant
Indexing
SS1 (mm) () () sin2
1
(mm)
38
19.0
38
19.0
0.11
45
22.5
45
22.5
0.15
66
33.0
66
33.0
0.30
78
39.0
78
39.0
0.40
83
41.5
83
41.5
0.45
97
49.5
97
49.5
0.58
113
56.5
113
56.5
0.70
118
59.0
118
59.0
0.73
139
69.5
139
69.5
0.88
168
84.9
168
84.9
0.99
FCC; wavelength=1.54056Å
Simple
BCC
Cubic
2 2
h22+k
sin
+l
2/
+l2 22/ Lattice
22+k
22+lh
22 2+k2sin
hsin
h22+k22+l22Parameter, a (Å)
0.11
3
0.15
4
0.30
8
0.40
11
0.45
12
0.58
16
0.70
19
0.73
20
0.88
24
0.99
27
21
0.037
42
0.038
63
0.038
84
0.036
5
10
0.038
6
12
0.036
8
14
0.037
9
16
0.037
10
18
0.037
11
20
0.037
0.055
0.11
0.038
0.75
0.050
0.10
0.050
0.10
0.045
0.09
0.048
0.097
4.023
3.978
3.978
4.039
3.978
Not
NotSimple
BCC
Cubic
4.046
But
what is the lattice
4.023
0.046
0.081
parameter?
4.023
0.049
0.088
4.023
0.050
0.09
4.023
Not Constant
0.050
0.0925
Constant; so it is FCC
Diffraction from a variety of materials
(From “Elements of X-ray
Diffraction”, B.D. Cullity,
Addison Wesley)
Reality
Crystallite size can be
calculated using
Scherrer Formula
t
0.9
B cos B
Instrumental broadening must be subtracted
(From “Elements of X-ray Diffraction”, B.D. Cullity, Addison Wesley)
Intensity of diffracted beam
•
•
•
•
•
•
polarization factor
structure factor (F2)
multiplicity factor
Lorentz factor
absorption factor
temperature factor
For most materials the peaks and their intensity are
documented
JCPDS
ICDD
Name and formula
Reference code:
00-001-1260
PDF index name:
Nickel
Empirical formula:
Ni
Chemical formula:
Ni
Crystallographic parameters
Crystal system:
Cubic
Space group:
Fm-3m
Space group number:
225
a (Å):
3.5175
b (Å):
3.5175
c (Å):
3.5175
Alpha (°):
90.0000
Beta (°):
90.0000
Gamma (°):
90.0000
Measured density (g/cm^3):
8.90
Volume of cell (10^6 pm^3):
43.52
Z:
4.00
RIR:
Status, subfiles and quality
Status:
Marked as deleted by ICDD
Subfiles:
Inorganic
Quality:
Blank (B)
References
Primary reference:
Hanawalt et al., Anal. Chem., 10, 475, (1938)
Optical data:
Data on Chem. for Cer. Use, Natl. Res. Council Bull. 107
Unit cell:
The Structure of Crystals, 1st Ed.
Stick pattern from JCPDS
http://ww1.iucr.org/cww-top/crystal.index.html
Actual Pattern
Lattice parameter, phase diagrams
Texture, Strain (micro and residual)
Size, microstructure (twins and
dislocations)
Bulk electrodeposited nanocrystalline nickel
Powder X-ray diffraction
is essentially a misnomer and should be replaced by
Polycrystalline X-ray diffraction
Information in a Diffraction Pattern
Phase Identification
Crystal Size
Crystal Quality
Texture (to some extent)
Crystal Structure
Intensity (a.u.)
Analysis of Single Phase
2(˚)
d (Å)
(I/I1)*100
27.42
3.25
10
31.70
2.82
100
45.54
1.99
60
53.55
1.71
5
56.40
1.63
30
65.70
1.42
20
76.08
1.25
30
84.11
1.15
30
89.94
1.09
5
I1: Intensity of the strongest peak
Procedure
Note first three strongest peaks at d1, d2, and d3
In the present case: d1: 2.82; d2: 1.99 and d3: 1.63 Å
Search JCPDS manual to find the d group belonging to the
strongest line: between 2.84-2.80 Å
There are 17 substances with approximately similar d2 but only 4
have d1: 2.82 Å
Out of these, only NaCl has d3: 1.63 Å
It is NaCl……………Hurrah
Specimen and Intensities
Substance
File Number
2.829 1.999 2.26x 1.619 1.519 1.499 3.578 2.668
(ErSe)2Q
19-443
2.82x 1.996 1.632 3.261 1.261 1.151 1.411 0.891
NaCl
5-628
2.824 1.994 1.54x 1.204 1.194 2.443 5.622 4.892
(NH4)2WO2Cl4
22-65
2.82x 1.998 1.263 1.632 1.152 0.941 0.891 1.411
(BePd)2C
18-225
Caution: It could be much more tricky if the sample is oriented or textured or your goniometer is not
calibrated
Presence of Multiple phases
d (Å)
I/I
4
1
More Complex
Pattern of Cu2O
Remaining
3.01 Lines 5
Several permutations combinations possible
2.47 I/I1
72
d
d (Å)
I/I1
e.g. d1; d2; and d3, the first three strongest lines (Å)
2.13
28
Observed
Normalized
3.020
9
show several alternatives
2.09 *
100
3.01
5
7
2.465 and
100 match
Then take any of the two lines together
1.80 *
52
20
2.47
72 1.50
100
It turns out that 1st and 3rd strongest
lies belong
2.135
37Patternto
for Cu
1.29
9
Cu and then all other peaks for Cu1.743
can be 1d (Å) 2.13I/I1
28
39
1.28 *
18
separated out
2.088
100
1.50
20
28
1.510
27
1.22
4
Now separate the remaining lines and normalize
1.808
46
9 1.08 * 13
20
1.287
171.278 1.29
the intensities
20
36
17
4 1.04 *
1.233
4 1.09the1.22
Look for first three lines and it turns
out that
0.98
5
1.0436
5
phase is Cu2O
0.98
5
7
1.0674
2
If more phases, more pain to solve0.9795
0.9038
3
0.91
4 0.8293
9
0.83
0.8083
8
0.81
*
*
8
10
Lattice Strain
do
No Strain
2
Uniform Strain
d strain
2
Non-uniform Strain
B roadeing b 2 2
d
d
2
tan
Texture in Materials
Grains with in a polycrystalline are not completely
randomly distributed
Clustering of grains about some particular
orientation(s) to a certain degree
Examples:
Present in cold-rolled brass or steel sheets
Cold worked materials tend to exhibit some texture after
recrystallization
Affects the properties due to anisotropic nature
Texture
Fiber Texture
A particular direction [uvw] for all grains is more or less parallel to
the wire or fiber axis
Double axis is also possible
e.g. [111] fiber texture in Al cold drawn wire
Example: [111] and [100] fiber textures in Cu wire
Sheet Texture
Most of the grains are oriented with a certain crystallographic plane
(hkl) roughly parallel to the sheet surface and certain direction [uvw]
parallel to the rolling direction
Notation: (hkl)[uvw]
Texture in materials
[uvw] i.e. perpendicular to
the surface of all grains is
parallel to a direction [uvw]
Also, if the direction [u1v1w1]
is parallel for all regions, the
structure is like a single
However, the direction
crystal
[u1v1w1] is not aligned for all
regions, the structure is like a
mosaic structure, also called
as Mosaic Texture
Pole Figures
(100) pole figures for a sheet material
(a) Random orientation (b) Preferred orientation
Thin Film Specimen
Grazing angle (very small, ~1-5)
B
B
Film or Coating
Substrate
Smaller volume i.e. less intensity of the scattered
beam from the film
Grazing angle
Useful only for polycrystalline specimens
Thin Film XRD
Precise lattice constants measurements derived from 2- scans, which
provide information about lattice mismatch between the film and the
substrate and therefore is indicative of strain & stress
Rocking curve measurements made by doing a q scan at a fixed 2
angle, the width of which is inversely proportionally to the dislocation
density in the film and is therefore used as a gauge of the quality of the
film.
Superlattice measurements in multilayered heteroepitaxial structures,
which manifest as satellite peaks surrounding the main diffraction peak
from the film. Film thickness and quality can be deduced from the data.
Glancing incidence x-ray reflectivity measurements, which can
determine the thickness, roughness, and density of the film. This
technique does not require crystalline film and works even with
amorphous materials.
Thin Films Specimens
B1B
(hkl) plane of the
substrate
B2
B
B1B2
Diffraction
from hkl
plane
i.e.
No Diffraction
from
hkl
occurs
plane
Single Crystal Substrate
If the sample and substrate is polycrystalline, then
problems are less
But if even if one of them is oriented, problems arise
In such situations substrate alignment is necessary
30
40
0028
0024
0026
0022
0020
0018
50
60
70
80
90
2216
20
10
△
△
SrTiO3 (110)
40
50
60
70
80
90
30
4016/
0416
20
10
014
△
△
Log Intensity (a.u.)
Bismuth Titanate thin
films on oriented
SrTiO3 substrates
Only one type of peaks
It apparent that films
are highly oriented
SrTiO3 (100)
*
△
0016
0012
0014
008
0010
006
004
Oriented thin films
△
SrTiO3 (111)
10
20
30
40
50
60
o
2 ( )
70
80
90
Degree of orientation
[uvw] corresponding
to planes parallel to
the surface
Film
Substrate
Side view
But what if the planes when looked from top have random orientation?
Top view
Pole Figure
SrTiO3 (110)
SrTiO3 (100)
SrTiO3 (111)
1
2
1
1
2
1
3
1
2
3
2
1
1
4 Peaks at ~50
Excellent in-plane
orientation
2 sets of peaks at ~ 5, 65
and 85°
Indicating a doublet or
opposite twin growth
2
2
1
3
2
2
3
3 sets of peaks
at ~ 35 and 85°
indicating a
triplet or triple
twin growth
(117) Pole Figures for Bismuth Titanate Films
Texture Evolution
3 (100) planes
inclined at 54.7°
to (110) plane,
separated
by
120°
Film
SrTiO3 (100)
Two (100) planes
inclined at 45° to (110)
plane in opposite
directions
BNdT(001)
STO [100]
STO(111)
45
54.7°
STO(110)
STO(111)
STO(100)
BNdT/SrTiO3 (100)
BNdT/SrTiO3 (110)
BNdT/SrTiO3 (111)
Rocking Curve
An useful method for evaluating the quality of oriented samples such as
epitaxial films
is changed by rocking the sample but B is held constant
Width of Rocking curve is a direct measure of the range of orientation present in
the irradiated area of the crystal
(0010) Rocking curve of (001)(2212) SrBi
Rocking
(116)oriented
film
2Ta2Ocurve
9 thin of
oriented SrBi2Ta2O9 thin film
Normal
B
FWHM = 0.07°
(a.u.)
Intensity
(a.u.)
Intensity
17.4
FWHM = 0.171°
32.432.4
17.5
32.6 32.832.8
17.6
17.7
() ()
17.6 32.6
17.8
33.0
18.0
33.0
17.833.233.2
18.2
Order Disorder Transformation
Structure factor is dependent on the presence
of order or disorder within a material
Present in systems such as Cu-Au, Ti-Al, NiFe
Order-disorder transformation at specific
compositions upon heating/cooling across a
critical temperature
Examples: Cu3Au, Ni3Fe
Order Disorder Transformation
Structure factor is dependent on the presence
of order or disorder within a material.
Complete Disorder
Example: AB with A and B atoms
randomly distributed in the lattice
Lattice positions: (000) and (½ ½ ½)
Atomic scattering factor
favj= ½ (fA+fB)
Structure Factor, F, is given by
F = Σf exp[2i (hu+kv+lw)]
= favj [1+e( i (h+k+l))]
= 2. favj when h+k+l is even
= 0 when h+k+l is odd
The expected pattern is like a BCC crystal
A
B
Order Disorder Transformation
Complete Order
Example: AB with A at (000) and B at (½ ½ ½)
Structure Factor, F, is given by
F = fA e[2i (h.0+k.0+l.0)]+ fA e[2i (h. ½+k. ½+l. ½)]
= fA+fB when h+k+l is even
= fA-fB when h+k+l is odd
The expected pattern is not like a BCC crystal,
rather like a simple cubic crystal where all the
reflections are present.
Extra reflections present are called as
superlattice reflections
A
B
Order-Disorder Transformation
Disordered Cu3Au
Ordered Cu3Au
Instrumentation
Diffractometer
Source
Optics
Detector
Incident Beam Part
Diffracted Beam Part
Sample
Source
Incident Beam
Optics
Diffracted Beam
Optics
Detector
Geometry and Configuration
Theta-Theta
Source and detector move θ, sample fixed
Theta-2Theta
Sample moves θ and detector 2θ , source fixed
Vertical configuration
Horizontal configuration
Incident Beam Part
Source Incident Beam
Optics
Horizontal sample
Vertical sample
Diffracted Beam Part
Sample Diffracted Beam
Optics
Detector
Sample translation
XYZ translation
Z translation
sample alignment
Sample exactly on the diffractometer circle
Knife edge or laser
Video microscope with laser
XY movement to choose
area of interest
X-ray generation
X-ray tube (λ = 0.8-2.3 Ǻ)
Rotating anode (λ = 0.8-2.3 Ǻ)
Liquid metal
Synchrotron (λ ranging from infrared to X-ray)
X-rays
X-ray tube
Be window
W cathode
Rotating anode
Electrons
Metal anode
Small angle anode
Small focal spot
Large angle anode
Large focal spot
Rotating anode of W or Mo for high flux
Microfocus rotating anode
Liquid anode for high flux
and small beam size
10 times brighter
100 times brighter
Gallium and Gallium, indium, tin alloys
Synchrotron provides intense beam but access is limited
Brighter than a thousand suns
Synchrotron
High brilliance and coherence
X-ray bulb emitting all radiations from IR to X-rays
http://www.coe.berkeley.edu/AST/srms
Moving charge emits radiation
Electrons at v~c
Bending magnet, wiggler and undulator
Straight section
wiggler and undulator
Curved sections
Bending magnet
Filter
to remove Kβ
For eg. Ni filter for Cu Kβ
Reduction in intensity of Kα
Choice of proper thickness
Slits
To limit the size of beam (Divergence slits)
To alter beam profile
(Soller slit angular divergence )
Narrow slits
Lower intensity
+
Narrow peak
Mirror
focusing and remove Kα2
Mono-chromator
Si
remove Kα2
Graphite
Beam Profile
Mirror
Parallel beam
Source
Soller slit
Detector
Mirror
Sample
Para-focusing
Detector
Sample
Source
Point focus
Detector
Sample
Source
Comparison
Parallel beam
Para-focusing
X-rays are aligned
X-rays are diverging
Lower intensity for bulk
samples
Higher intensity
Higher intensity for small
samples
Lower intensity
Instrumental broadening
independent of orientation
of diffraction vector with
specimen normal
Instrumental broadening
dependent of orientation of
diffraction vector with
specimen normal
Suitable for GI-XRD
Suitable for Bragg-Brentano
Texture, stress
Powder diffraction
Detectors
Single photon detector (Point or 0D)
scintillation detector NaI
proportional counter, Xenon gas
semiconductor
Position sensitive detector (Linear or 1D)
gas filled wire detectors, Xenon gas
charge coupled devices (CCD)
Area detectors (2D)
wire
CCD
3D detector
X-ray photon
Photoelectron or
Electron-hole pair
Photomultiplier tube or
amplifier
Electrical signal
Resolution: ability to distinguish between energies
Energy proportionality: ability to produce signal proportioanl to
energy of x-ray photon detected
Sensitivity: ability to detect low intensity levels
Speed: to capture dynamic phenomenon
Range: better view of the reciprocal space
Data collection and analysis
Choose 2θ range
Step size and time per step
Hardware: slit size, filter, sample alignment
Fast scan followed with a slower scan
Look for fluorescence
Collected data: Background subtraction, Kα2 stripping
Normalize data for comparison I/Imax
Summary
X-ray Diffraction is a very useful to characterize materials
for following information
Phase analysis
Lattice parameter determination
Strain determination
Texture and orientation analysis
Order-disorder transformation
and many more things
Choice of correct type of method is critical for the kind of
work one intends to do.
Powerful technique for thin film characterization