Podstawy Kriogeniki
advertisement

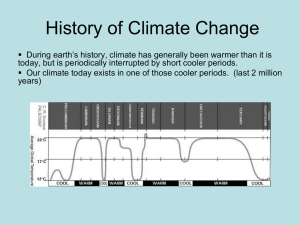
Refrigeration and cryogenics Zakład Kriogeniki i Technologii Gazowych Dr hab. inż. Maciej Chorowski, prof. PWr Methods of lowering the temperature Isentropic expansion Joule-Thomson expansion Free expansion – gas exhaust Gas isentropic expansion with external work T p1 p2 h1 1 h2 K 2' 2 S Gas isentropic expansion with external work Drop of the gas temperature: Entropy is a function of pressure and temperature S= S(p, T) Total differential must be equal to zero: S S dS dT dp 0 T p p T Differential effect of isentropic expansion ms shows the change in temperature with respect to the change of pressure: dT m s dp S S p T S T p Gas isentropic expansion with external work We know from thermodynamics S v T p p T cp S T p T dT m s dp S v T T p Tb cp cp We get where: b is coefficient of cubical expansion 1 b T p Gas isentropic expansion with external work For the ideal gas: ms After integration T2 p2 T 1 p1 1 T p 1 Piston expander 3 5 2 1 p1 4 6 p2 GAZ p1, T1, h1 GAZ p2, T2, h2 Cryogenic turboexpander GAZ p1, T1, h1 GAZ p2 T2 h2 Isenthalpic – Joule-Thomson - expansion When gas, vapour or liquid expands adiabatically in an open system without doing any external work, and there is no increment in velocity on the system reference surface, the process is referred to as throttle expansion. In practice, this process is implemented by installing in the gas stream some hydraulic resistance such as throttling valve, gate, calibrated orifice, capillary, and so on. 1 2 w1 w2 p p1 p2 q12 1 2 w2 w12 g z 2 z1 h2 h1 l12 2 Isenthalpic – Joule-Thomson - expansion T p1 p2 h 1 T1 T T2 2 h' K S Isenthalpic – Joule-Thomson - expansion Temperature drop in Isenthalpic – Joule-Thomson - expansion Enthalpy is a function of pressure and temperature: h= h(p, T) Total differential must be equal to zero: h h dh dp dT t p p T Differential throttling effect μh: dT m h dp h h p T h T p Isenthalpic – Joule-Thomson - expansion p, MPa 100,0 Ar 50,0 25,0 Ne 10,0 5,0 H2 2,5 powietrze N2 1,0 He 0,5 3 5 10 25 50 100 250 500 1000 T, K Isenthalpic – Joule-Thomson - expansion Gas Maximal inversion temperature, K eksperyment z równania van der Walsa Argon 765 ----- Azot 604 837 Hel – 3 39 ----- Hel – 4 46 34,3 Neon 230 ----- Powietrze 650 895 Metan 953 ----- Tlen 771 1090 204,6 223 Wodór Free expansion (exhaust) V0 T0,p0 V1 pf pf p f V2 pf Free expansion (exhaust) 1. 2. 3. 4. Adiabatic process Non equilibrium process – gas pressure and external pressure are not the same Constant external pressure (pf= const.) External work against pressure pf Free expansion (exhaust) Final gas temperature: I Law of Thermodynamics u f u0 p f (v f v0 ) where: u0, uf – initial and final gas internal energy v0, vf – initial and final gas volume Free expansion (exhaust) For ideal gas: u f u 0 c v (T f T0 ) p 0 v0 RT0 p f v f RT f cv R / 1 We get: 1 p f T f To T0 1 p0 T0 k T f 1 p f / p 0 k 1 Comparison of the processes for air Cryogenic gas refrigerators Heat exchangers Recuperative Regenerative Comparison of coolers Refrigerators with recuperative heat exchangers Joule – Thomson refrigerators Examples of miniature Joule-Thomson refrigerator Claude refrigerators Stirling coolers Stirling cooler Stirling cooler p p max In Stirling refrigerator a cycle consists of two isotherms and two isobars 2 T0 1 3 p min T0 q H2O 1 2 qH2O T q 4 T R R R R 3 4 q Stirling cycle is realized in four steps : 1. Step 1-2: Isothermal gas compression in warm chamber 2. Step 2-3: Isochoric gas cooling in regenerator 3. Step 3-4:Isothermal gas expansion with external work 4. Step 4-1: Isochoric gas heating in regenerator V2 V1 V T qH2O 2 T0 1 V2 V1 T 3 q 4 s Stirling split cooler Stirling cooler with linear motor Efficiency of Stirling cooler filled with ideal gas Str q T lc le T0 T v2 v2 dv l c RT o RT o ln v v1 v1 v1 dv v1 le RT RT ln v v2 v2 v2 q RT ln v1 Work of isothermal compression Work of isothermal expansion Heat of isothermal expansion Stirling cooler configuration: Stirling cooler used for air liquefact -ion Stirling cooler used for air liquefaction Two stage Stirling refrigerator Gifforda – McMahon cooler Gifforda – McMahon cooler Four steps of McMahon cycle: 1. Filling . 2. Gas displacement 3. Free exhaust of the gas 4. Discharge of cold chamber Efficiency of McMahon cooler: GM T p1 / p 2 1 To p1 / p 2 T ln p1 / p 2 McMahon refrigerator Combination of McMahon and J-T cooler, 250 mW at 2,5 K Pulse tube – free exhaust V0 T0,p0 V1 pf pf p f V2 pf Scheme of pulse tube cooler Development of pulse tube coolers Gifford, 1963, rather curiosity that efficient cooler Kittel, Radebaugh, 1983 orifice pulse tube Dr. Zhu et. al., 1994, multiply by-pass pulse tube Comparison of Stirling and orifice pulse tube cooler Pulse tube cooler for 77 K applications Weight:2.4 kg Dimensions (l x w x h):11.4 x 11.4 x 22 cm Capacity:2.5W @ 65K Ultimate low temperature:35K Input power2kW Pulse tube Two stage pulse tube Pulse tube configuration Adiabatic demagnetization of paramagnetic Paramagnetic salts Magnetic coolers Magnetic cooler Magnetic cooler with moving paramagnetic Three stage magnetic cooler with magnetic regenerator Ceramic magnetic regenerator material Gd2O2S with an average diameter of 0.35 mm for G-M and pulse tube cryocoolers. Cooler efficiency at 80 K „Family” of cryocoolers