Pathway and Systems Analysis

Biological pathway and systems analysis
An introduction
Biomedicine ‘after the human genome’
Patient
Molecular basis of disease
Current disease models
Molecular building blocks genes proteins very data-rich about genes, genome organisation, proteins, biochemical function of individual biomolecules
Patient
Physiology
Clinical data
Molecular basis of disease
Current disease models
Molecular building blocks genes proteins
Disease manifestation in organs, tissues, cells
?
Molecular organisation
Patient physiology, clinical data
Complex disease models tissues organs Computational modelling
Disease manifestation in organs, tissues, cells
Molecular building blocks genes proteins
Molecular organisation
Global approaches: Systems Biology
Perturbation
Living cell Dynamic response
Bioinformatics
Mathematical modelling
Simulation cell network modelling
“ Virtual cell ”
• Basic principles
• Applied uses, e.g. drug design
Dynamic biochemistry
• Biomolecular interactions
• Protein-ligand interactions
• Metabolism and signal transduction
• Databases and analysis tools
• Metabolic and signalling simulation
• Metabolic databases and simulation
• Dynamic models of cell signalling
Dynamic Pathway Models
• Forefront of the field of systems biology
• Main types
Metabolic networks
Gene networks
Signal transduction networks
• Two types of formalism appearing in the literature:
– data mining
e.g. genome expression at gene or protein level
contribute to conceptualisations of pathways
– simulations of established conceptualisations
Dynamic models of cell signalling
…from pathway interaction and molecular data
Erk1/Erk2 Mapk
Signaling pathway
…to dynamic models of pathway function
Schoeberl et al., 2002
Simulations: Dynamic Pathway Models
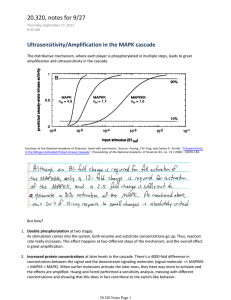
Epidermal growth factor (EGF) pathway • These have recently come to the forefront due to emergence of high-throughput technologies.
• Composed of theorised/ validated pathways with kinetic data attached to every biochemical reaction
- this enables one to simulate the change in concentrations of the components of the pathway over time given initial parameters.
• These concentrations underlie cell behaviour.
Schoeberl et al (2002) Nat. Biotech 20: 370
The epidermal growth factor receptor
(EGFR) pathway
The effect of the number of active EGFR molecules on ERK activation
EGFR
PLC Ras PI3K
PKC
ERK
TFs
MAPK PKB/Akt
Functional targets
CELL GROWTH AND PROLIFERATION
500,000 active receptors
50,000 active receptors =
Inhibition by one order of magnitude
Schoeberl et al ., 2002, Nat. Biotech. 20: 370
The effect of active EGFR number on ERK activation
500,000 active receptors
50,000 active receptors
Can this be achieved by receptor inactivation alone?
The effect of active EGFR number on ERK activation
50,000 active receptors with normal levels of
ERK or
ERK overexpression and cross-activation
Hunter and Borg (2003)
Virtual Physiological Human
Simulation of complex models of cells, tissues and organs www.vph-noe.eu
•Heart modelling: 40+ years of mathematical modeling of electrophysiology and tissue mechanics
•New models integrate molecular mechanisms and large-scale gene expression profiles
patient organ
Multi-level modelling integration across scales through computational modelling cell
Anatomy and integrative function, electrical dynamics
Vessels, circulatory flow, exchanges, energy metabolism
Cell models, ion fluxes, action potential, molecules, functional genomics
Spatial distribution of key proteins
• Transmural expression differences of an ion channel protein leads to different action potential profiles at the epicardium, midwall and endocardium
• Arrhythmias
Hunter et al (2005) Mechanisms of Ageing and Development
126:187 –192.
Virtual Physiological Human Project www.vph-noe.eu/
The Virtual Physiological Human https://www.youtube.com/watch?v=CM76-mS84Xs
The hallmarks of systems biology
formulate a general or specific question
define the components of a biological system
collect previous relevant datasets
integrate them to formulate an initial model of the system
generate testable predictions and hypotheses
systematically perturb the components of the system experimentally or through simulation
study the results
compare the responses observed to those predicted by the model
refine the model so that its predictions fit best to the experimental observations
conceive and test new experimental perturbations to distinguish between the multiple competing hypotheses
iterate the process until a suitable response to the initial question is obtained