File
advertisement

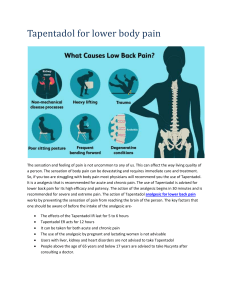
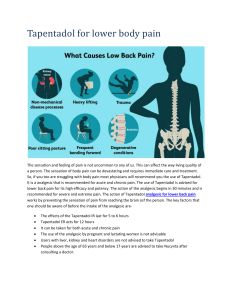
2011-2012 AMCP Pharmacy and Therapeutics Competition Ally Stojkoska September 24th, 2012 Outline What is a P&T Committee? What does a P&T Committee do? Overview of the P&T Competition Requirements for competing Benefits of competing This year’s competition topic Questions “Learning to work on a P&T committee through AMCP’s P & T competition is a skill that will be very useful in many pharmacist career pathways.” Vinson Lee, University of Southern California 2006 What is a P&T committee? P&T = Pharmacy and Therapeutics A committee that meets together to promote safe, effective, and cost-effective drug therapy Formulary management Primarily physicians and pharmacists Includes practitioners from a variety of specialties Develop policies regarding drug evaluation, selection, and utilization Who utilizes P&T Committee? Any organization that maintains a drug formulary utilizes a P&T Committee Hospitals Health Plans Prescription Benefit Managers (PBMs) VA and Military What do P&T Committees do? Improve the quality of patient care while controlling scarce healthcare resources What do P&T Committees do? Manage the development and maintenance of the organization’s drug formulary Review of scientific evidence Peer reviewed medical literature Clinical practice guidelines Pharmacoeconomic studies Perform cost-benefit analyses Weigh the drugs impact on patient populations Competition Overview Serve on a health plan’s P&T Committee Implement AMCP’s Format for Formulary Submissions Read the manufacturer’s dossier Research, Research, Research!!! Think in terms of real world experience Make a decision on a new drug Competition Requirements Work in a team of four students Requesting teams: Chose ONE person who you want to be in the same team with Must be an active AMCP member Guaranteed your ONE request Teams must be diverse and ideally contain a member from every year (P1,P2,P3,P4) Create your own diverse team- Must be OK’ed Competition Requirements Deliverables Evaluation of dossier / economic model Format will be provided Analysis of manufacturer’s value argument and data presented Evaluation advantages/disadvantages of this product versus currently available formulations and place in therapy Budget Impact Model Drug Monograph (maximum 15 pages) Competition Requirements Presentation Requirements 30 minute presentation and 30 minute Q&A session with judges Powerpoint Presentation All materials must be submitted and presentation completed to receive credit Competition Requirements All materials must be submitted and presentation completed to receive credit Benefits Teamwork Organization Learn/Improve skills Pharmacoeconomics Clinical evaluation Drug information Presentation skills More Benefits Networking opportunities Free food TWO ELECTIVE SEMESTER CREDIT HOURS Enhance your CV/Resume Winning team gets expenses paid to the 2012 AMCP Showcase Compete at national competition OSU Teams were placed in TOP 3 twice in the last seven years! Last year’s local winners competed at the national level. National Competition April 3-5 2013 in San Diego, Ca This year’s competition… Tapentadol extended-release tablets for oral use (NUCYNTA® ER) Tapentadol extended realease NUCYNTA ER is an extended release opioid agonist indicated for the management of moderate to severe chronic pain in adults and neuropathic pain associated with diabetic peripheral neuropathy in adults Sign up for P&T Competition! Meet with your team regularly Try to work ahead of the schedule – You can start your research on Tapentadol NOW! Do not downplay the importance of the written materials Only written material gets sent for national consideration Do not be afraid to ask for direction Faculty cannot give you direct answers, but may point you in the right direction Important Dates Team and individual sign-up Email OSUPTCOMP@gmail.com Ally (stojkoska.1@osu.edu) Competition date: 4th week of Jan 2012 National Competition date: April 2012 Questions??? Contact Ally (stojkoska.1) or Jina (park.903)