NHS Sickle Cell & Thalassaemia Screening Programme
advertisement

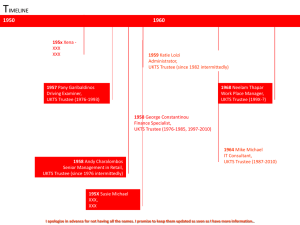
United Kingdom Thalassaemia Society 1976 – Present Day Running a Successful Society To empower those affected by or working with thalassaemia and to be their definitive source of information, education and research Mission Statement Copyright UKTS 2011 2 25 November 2011 1. To encourage promote and support all areas of prevention and to allow accurate, informed choice. 2. To create and maintain the highest standard of care for thalassaemics. 3. To encourage, promote and support all thalassaemics in socially integrating and achieving their full potential. 4. To encourage, promote and support research in the treatment and care of thalassaemia. 5. To resource the UKTS to fulfil, sustain and grow it activities in the above areas. 6. To work towards a cure for Thalassaemia. The Aims of a Society Copyright UKTS 2011 3 25 November 2011 Organizational Structure of the UKTS The Management Committee PRESIDENT SECRETARY TREASURER ASSISTANT TREASURER VICE PRESIDENT BOARD MEMBER BOARD MEMBER BOARD MEMBER Management Committee Structure Copyright UKTS 2011 5 25 November 2011 Research and Education Over the past 35 years the UKTS has donated more than £2 million pounds to research. Although all of our projects are important we above all try to fund those that stand out head and shoulders above the rest, and that can help change the lives of thalassaemics all over the UK and world. £5,000 contribution to the development of the Syringe Driver (pump) in 1976. Investing in the development of the oral chelator, Deferiprone (L1), funded and supported by the UKTS to the tune of £ 750,000. £27,500 for Growth and Development Research. Research into prevalence, transmission and treatment of Hepatitis C (£54,000 in 1990) Support for Bone Disease research and treatment developments. Funding for thalassaemia patients’ register to 2002. Research Funding Copyright UKTS 2011 7 25 November 2011 Development and set up of the first ever Pre Natal Diagnosis screening, Dr M. Petrou, UCLH, £30,000 in 1979. Development of The National Standards For The Clinical Care Of Children And Adults With Beta Thalassaemia in 2005 and 2008. Funding research into prognostic value of MRI imaging £33,600. Creation and distribution of individual Patient Held Record. Targeted workshops, doctors and patients conferences every year, (£20,000-30,000). Assessment of novel beta globin transcription units within retroviral vectors for gene therapy (£60,000 in 2000-3) Research Funding cont. Copyright UKTS 2011 8 25 November 2011 Pre-implantation genetic diagnosis for thalassaemia £64,940 Dr M. Petrou, UCLH, 2003. £70,000 – development of an in utero approach for thalassaemia gene therapy , Dr M. Antoniou & Dr S. Waddington, KCL, 2008. £35,000 – development & characterisation of zinc finger nucleases specific for the human beta globin gene, Dr A. Porter & Prof I. Roberts ICL, 2009. Commissioned research to study psychosocial issues and impacts affecting the transition from childhood to adulthood of adolescents with Beta-Thalassaemia, Dr. S. Dyson, de Montfort University, 2010 Research Funding cont. Copyright UKTS 2011 9 25 November 2011 • £ 289,478 Asian Awareness Campaign in 1997/2000 to increase awareness of the condition amongst the UK Asian Communities and improve their INFORMED CHOICE available for prevention to the individuals and couples in these communities who are planning a family. £ 20,000 on education of at risk communities, through ethnic radio, television, magazines, newspapers and website (per year) £ 20,000 Website and publications. £ 20,000 – £ 30,000 Targeted workshops and patients conference (per year) £ 16,000 for the Patient Held Record Education Funding Copyright UKTS 2011 10 25 November 2011 £ 3,000-£ 5,000 Annual support grants for promising doctors to attend TIF’s e-MSc course on Haemoglobinopathies at UCL. Development of 3 DVDs for increasing awareness about screening, and treatment in the at risk community and the general population. Development and implementation of a 3 year North England Awareness Project in association with the NHS SC&T Screening Programme for outreach and community work carried out by the UKTS focusing on communities affected by Thalassaemia in the north of England. Education Funding cont. Copyright UKTS 2011 11 25 November 2011 NHS Sickle Cell & Thalassaemia Screening Programme The NHS Sickle Cell & Thalassaemia Screening Programme aims to establish high quality newborn screening programmes for sickle cell disorders and antenatal screening programmes for sickle cell and thalassaemia. Members of Steering Committee and all subcommittees. National Standards Clinical Care of Patients with Beta Thalassaemia 2005 (revised in 2008) Co-authored by a multi-professional group and designed to address the concerns that clinical services though of a high standard were not consistent around the country. Have become a leading document for clinicians of UK and international model. Leading Member of the UK Dept. of Health Clinical Service Development Group and The National Haemoglobinopathies Project Created to develop the vision for integrated, equitable and effective haemoglobinopathy lifespan care and to implement it across England. High Level Participation Copyright UKTS 2011 12 25 November 2011 Permanent Member of EURORDIS (European Rare Diseases Organisation). UKTS Expert patients contribute their knowledge and expertise in many fields to help other patients with Rare Conditions in the EU Permanent Member of EPPOSI. A collaborative not-for-profit, partnership-based multi-stakeholder think tank based in Brussels, to develop workable European Models for Chronic Conditions Management, HTAs, Rare Diseases and foster Innovation. Founding and long term Board Member of TIF. UKTS expert patients have been continuously contributing in all areas of TIF projects, initiative and activities as well as on many delegation missions to countries around of the world. Taken part in meetings with Government Ministries, Health Authorities, clinicians, patients and parents and have assisted many countries in developing better Healthcare and effective Patient associations. High Level Participation cont. Copyright UKTS 2011 13 25 November 2011 Regular contributor to international EU conferences on Healthcare Policy, Development, Regulation, Access to Medications, Health Technology Assessments and their impact. Advisors to the European Medicines Agency (EMA) As the EU’s Drug Regulatory Authority we advise their Scientific Advice W.P. for Drug licensing and approval of new upcoming medicines relevant to thalassaemia. Member of UK All Parliamentary Party Group for Sickle Cell and Thalassaemia regularly meeting and lobbying senior parliamentarians in both Houses of Lords and Commons to improve patient services and conditions. High Level Participation cont. Copyright UKTS 2011 14 25 November 2011 Advisors to the National Institute of Clinical Excellence (NICE) Advising them on the remit, scope and criteria, of their assessment for access by patients to all chelators on the NHS. Involved in the 18 month evaluation for chelator efficacy and economic benefit driving home the importance and benefits to the patients having free access to all 3 medications. Founding and Board Member of the National Haemoglobinopathies Register (NHR) An NHS wide database of patient events, and outcomes aiming to a monitor patients with red cell disorders in the UK. It collects data required by the Department of Health from haemoglobinopathy centres aiming to optimise and to improve patient care, nationwide. High Level Participation cont. Copyright UKTS 2011 15 25 November 2011 Founding and Board Member of the UK Forum of Haemoglobinopathies Member of this professional organisation of doctors working on HbOs contributing the patient view on the peer review of children's care services in hospital, future policy implementation, ensuring UKTS Standards of Care are implemented nationwide. Board Member of the Genetic Alliance Group A national charity of patient organisations with a membership of over 140 charities supporting all those affected by genetic disorders; aiming to improve the lives of people affected by genetic conditions by ensuring that high quality services and information are available to all who need them. High Level Participation cont. Copyright UKTS 2011 16 25 November 2011 All mature societies at some point will need to re-evaluate their long term strategy. As treatment, quality of life and life expectancy increases then we find that a society will need to continue research as thalassaemia evolves from now as a condition. However we also need to recognise that there will be a shift towards the following: Attentions to the needs of an older thalassaemic population. More demand and pressure by the developing world for a cure. Effective parliamentary/health service lobbing. New challenges arising i.e. Personalised Medicine treatments. The Future.... is ours to reach it Copyright UKTS 2011 17 25 November 2011 At both local and international levels we need not just to continue the work that we do but to also provide a global strategy for all society : One standard worldwide, a common fund, open mindedness to new treatments and ideas. Genetic Research into curing Thalassaemia Optimising Treatment & Protocol Standards Effect care delivery that allows patients their full economic activity and education. By looking after ourselves, by questioning, by taking responsibility for our health and our treatment, by focusing on the positive elements of thalassaemia we become the informed patient. By becoming the informed and expert patient we increase our survival rate and achieve “normality”. The Future....is ours to reach it Copyright UKTS 2011 18 25 November 2011 THANK YOU e-mail : office@ukts.org website : www.ukts.org 19 Copyright UKTS 2011 25 November 2011