
Journal Club
Sidharth Bagga MD
Cytisus laborium L. (Golden rain acacia)
Case
CC: “I’d like to quit, but it is cheaper to keep smoking”
56 male with 20 pack year smoking history
Seen in clinic in follow up
Has had 4 unsuccessful attempts at quitting smoking
Hx of HTN
Patient is a construction worker and not very eager to spend his beer
money on smoking cessation treatment anymore
So what do you do now?
1.
2.
3.
4.
5.
Smoking cessation tricks (impotence, wrinkles, etc)
Ask, document and move on
Varencycline
Nicotine replacement
New non approved Cytisine
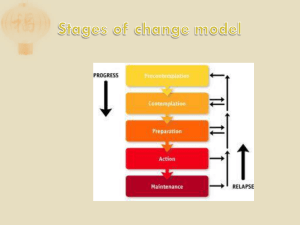
Background / Context?
Smoking Prevalence
Tobacco use remains chief avoidable cause of death in US
1960s – 42% to 2010s – 20%
Many clinicians do not offer smoking cessation
Only 20% of smokers are ready to attempt quit at any time
95% of unaided attempts fail
Pts typically consume 50% of recommended dose of medications
Only complete about half of smoking cessation counseling
Treatment availability / ease of use?
If treatment delayed or separate location, only 10% follow through
If same location and immediate, 1/3rd follow through
Schweikert et al, Lancet, 2009
Cytisine
Nicotine
Journal Club
Research Question
Assess Cytisine’s
efficacy and safety in a context that could be
replicated globally
relatively short treatment goal (25 days)
minimal contact with health professionals.
Design / Subjects
Prospective, randomized, single center, doubleblind trial between 12/2007 & 9/2010 in Poland.
Inclusion Criteria
Exclusion Criteria
Adults smoke > 10 cigs/day + willing to stop
permanently, literate, provide consent
Pregnancy, breast feeding, current psychiatric d/o,
medical contraindication for Cytisine (arterial HTN +
advanced arteriosclerosis)
Sampling
No other smoking cessation drugs current
Relapse: > 5 cigs used since enrollment, no smoking in last
week, breath CO < 10ppm
Study Procedures
Variable block randomization (eliminates early/late bias)
First Visit
6 months
Age, sex, employment, marital status, Nicotine Dependence
(FTND), # of cigs/day, duration of smoking, & quit attempts
Beck Depression Inventory
Phone contacts
If abstinent: return to clinic, CO in exhaled breath, Depression
12 months (same)
Adverse Reactions
Asked, if ‘Yes’ then verbatim copied and compiled according to
standard adverse reaction data
Measurements - Outcomes
Primary Outcome
Secondary Outcome
12 month of abstinence from smoking
Changed from original 6 months before unblinding/data
analysis
Abstinence at 6 months and point prevalence at 12
months (week before visit)
Criteria for abstinence
Fewer than 5 cig in last 6 months
Confirmed with less than 10 ppm of CO in exhaled
breath
Statistical Analysis
Sample Size
Calculated need for 740 pts to show a difference of 6
points b/w groups to show percentage reduction in
abstinence with 80% power & p < .05
Intention to treat analysis
Categorical variables – x2 and Fischer Exact test
Logistic regression to examine efficacy with adjustment
from baseline characteristics
P < .05 was considered statistically significant
Study Enrollment & Follow Up
Baseline Characteristics
Cytisine administration
DAY 1-3
DAY 4-12
DAY 13-16
DAY 17-20
DAY 20-25
5 tabs/day
4 tabs/day
6 tabs/day
QUIT DATE
3 tabs/day
2 tabs/day
Total: 101 tablets
Study Procedures
Results
Adverse Events
Discussion
Evidence of efficacy of Cytisine as an aid in
smoking cessation
Relative Rate
More gastrointestinal adverse events
Rate of discontinuation / dose reduction (same as placebo)
Cytisine (3.4) higher than Varenicline (2.3) & Nicotine (1.6)
Absolute rate of Abstinence
Lower than varenicline, but similar to nicotine replacement
Treatment period (4, 6, 8, 12 weeks)
May reduce cravings & make cigs less satisfying, like
Varenicline (a2b4 receptor)
Limitations
Adults with previous attempts? Which medications?
Outcomes at 12 weeks? Standard for other drug regimens
Need to compare to current standard of care
Conclusion
Cytisine needs FDA approval
Studies to assess efficacy in conjunction with
cognitive feedback
Cheap, effective alternative
JAMA Article Assesment: Therapy
Are the results of the study valid?
Was the assignment of patients to treatments randomized? Yes
Were all pts who entered the trial properly accounted for and attributed at its
conclusion? Yes
Was follow-up complete? Yes
Were pts analyzed in the groups to which they were randomized? Yes
Were patients, health workers, and study personnel blind to treatment? Yes
Were the groups similar at the start of the trial? Yes
Aside from the experimental intervention, were the groups treated equally? Yes
JAMA Article Assesment: Therapy
What were the results?
How large was the treatment effect?
How precise was the estimate of the treatment effect?
Will the results help me in caring for my patients?
Can the results be applied to my patient care? Yes (bulgarian drugs)
Were all clinically important outcomes considered? SOME
Are the likely treatment benefits worth the potential harms and costs?
Yes







![[Section 2.0] PICO Question Table – LC III Guidelines](http://s3.studylib.net/store/data/006838942_1-d31e4d679d8742f590f21898872c27c9-300x300.png)
