Clinical Management Plans Workshop
advertisement

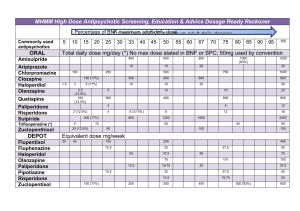
When to use supplementary prescribing and How to write a clinical management plan Kimberley Tordoff Introduction • On completion of this course you will gain a qualification of being an IP & SP • Therefore you must demonstrate the ability to complete a clinical management plan required for supplementary prescribing • Errors on your clinical management plan (CMP) may lead you to fail your portfolio Uses of CMP • Long term conditions • Useful if working in a new area • Useful for prescribing Controlled Drugs (CDs) • Widely used before the whole BNF opened CMPs • Blank templates on Department of Health website • You may use your own version as long as ALL the legally required information is there • Choose the one appropriate to your practice (do you have access to the notes or not?) • Only put one condition to be treated or one symptom • For CMPs to work they need to be quick and simple (NPC 2003) CMPs • You are the SP and the doctor is the IP • If more than one SP all must be listed by name and all must sign in the relevant section to enable more than one SP in a team to use the plan. • Use the patient details & address in your module guide • Fill in the patient details including an NHS number (false) • Condition to be treated • Aim of the treatment; be specific e.g. BP between x & x or Hba1c @ x, peak flow over x. CMPs • Preparation can be the name of 1 drug if it is that specific, but a plan works better if it is over a range of drugs or the whole class of drugs • Include the section of the BNF e.g. Section 3.2.1of BNF vol 61 2011 (Joint Formulary Committee, 2011). • If you are experienced in this area then you would want a range of drugs • Dose – range of dose to prescribe for a specific drug or as per section 3.2.1. of BNF vol 61 2011 (Joint Formulary Committee, 2011). CMPs • Reasons for referral back to IP • Be specific and not vague-if you put ‘side effects’ then you would have to refer back for every single slight problem • Think about this in reality • BP above x on x occasions despite maximum dose • Cough on ace may not be needing to do back to see IP if you have given yourself a wide enough section of BNF so you can try an alternative Guidelines & Protocols • Must include BNF with volume number and date • Guidelines referenced properly • These can be local as long as they are recognised and robust Review & Record • Frequency of review is a heading and must be completed • Put a time frame for IP & SP • IP must be at least yearly • SP must be more frequent than IP • Whatever the shared record is then document it e.g. hospital notes, EMIS. Adverse Drug Reactions (ADRs) • • • • • • Inform IP Document in patients notes Inform MHRA via yellow card system Signatures are needed NOT typed names Dated Patients signature is not required but document in notes that they have given informed consent • When you write out your prescription the pharmacist you must know that you are prescribing within a CMP so write SP after your name Conclusion • This is a fictitious CMP so don't worry that you are signing something illegally • Get this done soon whilst it is fresh in your mind References Joint Formulary Committee British National Formulary (61) Ed. London: British Medical Association and Royal Pharmaceutical Society (2011) National Prescribing Centre (2003) (NPC) Supplementary Prescribing A resource to help healthcare professionals to understand the framework and opportunities http://www.npc.nhs.uk/resources/healthcare_re source.pdf This work was produced as part of the TIGER project and funded by JISC and the HEA in 2011. For further information see: http://www.northampton.ac.uk/tiger. This work by TIGER Project is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. Based on a work at tiger.library.dmu.ac.uk. The TIGER project has sought to ensure content of the materials comply with a CC BY NC SA licence. Some material links to third party sites and may use a different licence, please check before using. The TIGER project nor any of its partners endorse these sites and cannot be held responsible for their content. Any logos or trademarks in the resource are exclusive property of their owners and their appearance is not an endorsement by the TIGER project.