Note for critical appraisal - Penn Medicine
advertisement

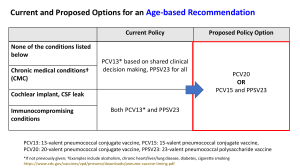
Developing a Vaccine against Pneumonia, “The Captain of Death” -- Osler History • 1881 Louis Pasteur discovers the pneumococcus bacterium • 1886 Gold mining begins in South Africa • 1895 Pneumococcus identified as the cause of epidemic pneumonia that kills 35% of miners who are infected • 1904 Miners strike for health reasons, including pneumonia • 1910 Treatment with ethylhydrocuprein, a quinine derivative, is found to be too neurotoxic • 1911 Mine owners hire the developer of typhoid fever vaccine to develop a pneumococcal vaccine Important Early Vaccine Studies Date Vaccine composition Trial type 1914 Whole cell, heat-killed organisms Prospective, placebocontrolled, non randomized trial with 1 yr follow-up Number of participants 50,000 South African miners Result Not reported, but vaccine given to all miners Therapies Other Than Vaccine • 1920s through 1940s antiserum – Gram stain sputum to confirm cause – Identify strain (Neufield reaction) – Give strain-specific horse or rabbit antiserum intravenously – Problems included severe allergic reactions and prolonged delays identifying strain and obtaining antiserum for treatment • 1938 Sulfapyridine – Problems included vomiting and severe rash Therapies Other Than Vaccine • 1920s through 1940s antiserum – Gram stain sputum to confirm cause – Identify strain (Neufield reaction) – Treat with strain-specific horse or rabbit antiserum intravenously – Problems included severe allergic reactions and prolonged delays identifying strain and obtaining antiserum for treatment • 1938 Sulfapyridine – Problems included vomiting and severe rash Next Major Vaccine Study Date Vaccine composition 1944 Polysaccharide types 1,2,5,7 made by E.R.Squib & Sons Trial type Placebo-controlled, randomized with 6 mo followup Number of participants 17,000 members of the US Air Force Result 90% effective More History • 1941 First reported use of penicillin to treat pneumonia in humans • 1945 FDA approves penicillin • 1946 FDA approves Squibb vaccine • 1951 Squibb stops making its vaccine because no one is using it The Penn Connection Robert Austrian, MD, 1917-2007 http://www.historyofvaccines.org/content/robert-austrian-1 • Austrian suspected that serious risks from pneumococcal infection persisted despite the prevalence of antibiotics, and he produced the evidence needed to persuade a skeptical medical community. After a study of patients in New York City’s Kings County Hospital from 1952 to 1962, Austrian concluded that the incidence of pneumococcal pneumonia was much higher than was thought at the time and that the mortality rate of 15% in bacteremic cases was unchanged, despite antimicrobial treatment. • In 1962, Austrian left the State University of New York College of Medicine at Brooklyn to join the University of Pennsylvania. There, he developed a new vaccine and conducted clinical trials among gold miners in South Africa that found the vaccine safe and efficacious. • The recent emergence of widespread resistance of pneumococcus to commonly used antibiotics highlights the incredible importance of the vaccine. What he did to solve a major human disease problem, often almost totally by himself, is extremely rare in modern medicine. – Paraphrased from the obituary by Harvey Freidman in JCI Current Vaccines • Pneumococcal conjugate vaccine – Polysaccharides conjugated with diphtheria proteins to enhance immunogenicity – Active against 13 strains – Abbreviated PCV13 • Pneumococcal polysaccharide vaccine – Not conjugated – Active against 23 strains – Abbreviated PPSV23 Current Vaccine Recommendations for Infants, Toddlers, and Children Pneumococcal conjugate vaccine (PCV13) is recommended for all infants, toddlers, and children from 2 months through 5 years of age. It also is recommended for children 6 through 18 years of age with certain medical conditions regardless of whether they have previously received a pneumococcal vaccine. Current Vaccine Recommendations for Adults Pneumococcal polysaccharide vaccine (PPSV23) is recommended for adults 65 years and older; for younger adults who have a chronic illness or who smoke; for Alaska Natives and certain American Indian populations; for people who had their spleen removed; and for those with weakened immune systems. Important Observations • In elderly adults, PPSV23 protects against invasive pneumococcal disease (IPD) but perhaps not against nonbacteremic pneumococcal disease (NPD) • In children, PCV13 protects against IPD and NPD • The effect on adult disease from childhood vaccination is unknown Cost-effectiveness of Adult Vaccination Strategies Using Pneumococcal Conjugate Vaccine Compared With Pneumococcal Polysaccharide Vaccine JAMA. 2012;307(8):804-812 What Do We Know about the Journal and the Authors? Vaccination Strategies Modeled • • • • • • No vaccination PPSV23 at age 65 (current recommendation) PCV13 at age 65 PCV13 at age 50, and PPSV23 at age 65 PCV13 at age 50 and again at age 65 PCV13 at age 50 and again at age 65 plus PPSV23 at age 75 Model Features • • • • Life-time horizon Societal perspective 3% discount rate for costs and benefits Quality of life measured on 0 to 1 scale, and QALYs calculated by multiplying the utility of a state times the duration in that state Model Inputs from Table 1 • • • • • Vaccine effectiveness Vaccine adverse events Disability – risk and mortality Utility weights by age and level of risk Hospitalization rates and costs by type of disease (IPD vs. NPD) • Vaccine costs Results in Table 3 Henry’s Efficient Algorithm Results in Table 3 Deterministic Sensitivity Analyses One-Way Only More Results in Table 3 How to Describe the Results of Probabilistic Sensitivity Analyses: Could these results have occurred by chance alone? • Plot results of separate Monte Carlo runs as a cloud in the cost-effectiveness plane • Calculate p-values or confidence intervals • Create cost-effectiveness acceptability curve Step 4. Analyze the “Stochastic” Tree Sampling Cost-Effectiveness Plane Incremental CE Plot Report QUADRANT INCR EFF INCR COST C1 IV IE>0 IC<0 Superior C2 I IE>0 C3 III C4 FREQ PROPORTION 0 0 IC>0 ICER<50k 106 0.0212 IE<0 IC<0 ICER>50k 0 0 I IE>0 IC>0 ICER>50k 1 2.00E-04 C5 III IE<0 IC<0 ICER<50k 0 0 C6 II IE<0 IC>0 Inferior 4893 0.9786 origin IE=0 IC=0 0/0 0 0 Indiff Do You Agree with the Authors’ Conclusion? Overall, PCV13 vaccination was favored compared with PPSV23, but the analysis was sensitive to assumptions about PCV13 effectiveness against nonbacteremic pneumococcal pneumonia and the magnitude of potential indirect effects from childhood PCV13 on pneumococcal serotype distribution. My Translation of the Authors’ Conclusion In the base case analysis and in most of the deterministic sensitivity analyses, PCV13 vaccination was favored compared with PPSV23, but the analysis was sensitive to assumptions about PCV13 effectiveness against nonbacteremic pneumococcal pneumonia and the magnitude of potential indirect effects from childhood PCV13 on pneumococcal serotype distribution. Two Problems with This Conclusion In the base case analysis and in most of the deterministic sensitivity analyses, PCV13 vaccination was favored compared with PPSV23, but the analysis was sensitive to assumptions about PCV13 effectiveness against nonbacteremic pneumococcal pneumonia and the magnitude of potential indirect effects from childhood PCV13 on pneumococcal serotype distribution. 1. This conclusion does not help readers decide whether there is a preferred vaccine strategy, and if so, which one it is. 2. This conclusion ignores the probabilistic sensitivity analysis. What about this Alternative Conclusion? In the base case analysis and in most of the deterministic sensitivity analyses, the strategy of giving PCV13 at age 50 and again at age 65 was favored over other strategies, but our probabilistic sensitivity analysis showed that the differences between this strategy and other strategies could have occurred by chance alone.